Abstract
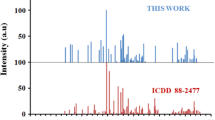
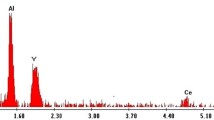
Garnet phosphor Y3Al5O12:Ce3+ is prepared in the Y2O3–Al metal–CeO2 ternary system by the solid-state reaction method in the air. For the first time, metal Al is used as a source of aluminum for the reaction instead of traditional oxide Al2O3. It is shown that the chemical reaction can be realized at lower temperatures and without use of special reducing atmosphere. The structural and spectroscopic properties of the prepared powder phosphor are very close to those earlier reported for the Y3Al5O12:Ce3+ single crystal.






Similar content being viewed by others
References
Geller S (1967) Crystal chemistry of the garnets. Z Kristallogr 125:1–47
Hofmeister AM, Chopelas A (1991) Vibrational spectroscopy of end-member silicate garnets. Phys Chem Miner 17(6):503–526
Liou JG, Ernst WG, Zhang RY, Tsujimori T, Jahn BM (2009) Ultrahigh-pressure minerals and metamorphic terranes—the view from China. J Asian Earth Sci 35(3–4):199–231
Jüstel T, Nikol H, Ronda C (1998) New development in the field of luminescent materials for lighting and displays. Angew Chem Int Ed 37:3084–3103
Huber G, Kränkel C, Petermann K (2010) Solid-state lasers: status and future. J Opt Soc Am B 27(11):B93–B105
Speghini A, Piccinelli F, Bettinelli M (2011) Synthesis, characterization and luminescence spectroscopy of oxide nanopowders activated with trivalent lanthanide ions: the garnet family. Opt Mater 33:247–257
Chen Feng (2012) Micro- and submicrometric waveguiding structures in optical crystals produced by ion beams for photonic applications. Laser Photon Rev 6(5):622–640
Sangheta Jasbinger, Kim Woohong, Villalobos Guillermo, Shaw Brandon, Baker Colin, Frantz Jesse, Sadowski Bryan, Aggarwal Ishwar (2013) Ceramic laser materials: past and present. Opt Mater 35:693–699
Song Zhen, Liao Jing, Ding Xianlin, Liu Xiaolang, Liu Quanlin (2013) Synthesis of YAG phosphor particles with excellent morphology by solid state reaction. J Cryst Growth 365:24–28
Xia Zhiguo, Liu Quanlin (2016) Progress in discovery and structural design of color conversion phosphors for LEDs. Prog Mater Sci 84:59–117
Xia Z, Meijerink A (2017) Ce3+-doped garnet phosphors: composition modification, luminescence properties and applications. Chem Soc Rev 46:275–299
Kidyarov BI, Atuchin VV (2007) Universal crystal classification system “point symmetry—physical property”. Ferroelectrics 360:96–99
Bagdasarov KhS, Bolotina NB, Kalinin VI, Karyagin VF, Kuzmin BV, Myradyan IA, Ryadnov SN, Uyukin EM, Chernaya TS, Fedorov EA, Chudakov VS, Simonov VI (1991) Photoinduced effects and real structure of crystals of yttrium aluminum garnet. Kristallografiya 36:715–728
Ozawa Tadashi C, Kang Sung J (2004) Balls & sticks: easy-to-use structure visualization and animation program. J Appl Cryst 37:679
Aleksandrovskii AS, Bezmaternykh LN, Gudim IA, Krylov AS, Temerov VL (2002) Optical spectra of Gd3Ga5O12: Mn crystals. Inorg Mater 38(10):1032–1034
Kaminskii AA, Butashin AV, Aleksandrov KS, Bezmaternykh LN, Temerov VL, Gudim IA, Kravtsov NV, Firsov VV, Seo DT, Hömmerich U, Temple D, Braud A (2002) Gd3Ga5O12:Nd3+ crystals for a continuous-wave diode-pumped laser operating in 4F3/2 → 4I11/2 and 4F3/2 → 4I13/2 channels. Cryst Rep 47(2):308–312
Lu JR, Ueda K, Yagi H, Yanagitani T, Akiyama Y, Kaminskii AA (2002) Neodymium doped yttrium aluminum garnet (Y3Al5O12) nanocrystalline ceramics—a new generation of solid state laser and optical materials. J Alloys Compd 341(1–2):220–225
Boyer JC, Vetrone F, Capobianco JA, Speghini A, Bettinelli M (2004) Yb3+ ion as a sensitizer for the upconversion luminescence in nanocrystalline Gd3Ga5O12:Ho3+. Chem Phys Lett 390:403–407
Aleksandrovsky AS, Arkhipkin VG, Bazmaternykh LN, Gudim IA, Krylov AS, Vagizov F (2008) Origin of color centers in the flux-grown europium gallium garnet. J Appl Phys 103:083102
Luo Y, Xia Z (2014) Effect of Al/Ga substitution on photoluminescence and phosphorescence properties of garnet-type Y3Sc2Ga3−xAlxO12:Ce3+ phosphor. J Phys Chem C 118:23297–23305
Atuchin VV, Beisel NF, Galashov EN, Mandrik EM, Molokeev MS, Yelisseyev AP, Yusuf AA, Xia Z (2015) Pressure-stimulated synthesis and luminescence properties of microcrystalline (Lu, Y)3Al5O12:Ce3+ garnet phosphors. ACS Appl Mater Interfaces 7(47):26235–26243
Zhou Wei, Ma Xinxu, Zhang Manli, Luo Yi, Xia Zhiguo (2015) Synthesis and photoluminescence properties of green-emitting Lu3(Al, Sc)5O12:Ce3+ phosphor. Ceram Int 41:7140–7145
Ji H, Wang L, Molokeev MS, Hirosaki N, Xie R, Huang Z, Xia Z, ten Kate OM, Liu L, Atuchin VV (2016) Structure evolution and photoluminescence of Lu3(Al, Mg)2(Al, Si)3O12:Ce3+ phosphors: new yellow-color converters for blue LED-driven solid state lighting. J Mater Chem C 4(28):6855–6863
Atuchin VV, Grivel J-C, Korotkov AS, Zhang Z (2008) Electronic parameters of Sr2Nb2O7 and chemical bonding. J Solid State Chem 181:1285–1291
Atuchin VV, Gavrilova TA, Grivel J-C, Kesler VG, Troitskaia IB (2012) Electronic structure of layered ferroelectric high-κ titanate Pr2Ti2O7. J Solid State Chem 195:125–131
Ji Haipeng, Huang Zhaohui, Xia Zhiguo, Molokeev Maxim, Atuchin Victor V, Fang Minghao, Huang Saifang (2014) New yellow-emitting whitlockite-type structure Sr1.75Ca1.25(PO4)2:Eu2+ phosphor for near-UV pumped white light-emitting devices. Inorg Chem 53(10):5129–5135
Atuchin VV, Aleksandrovsky AS, Chimitova OD, Gavrilova TA, Krylov AS, Molokeev MS, Oreshonkov AS, Bazarov BG, Bazarova JG (2014) Synthesis and spectroscopic properties of monoclinic α-Eu2(MoO4)3. J Phys Chem C 118(28):15404–15411
Zhang Haiming, Zhang Haoran, Liu Weiren, Liu Yiangliang, Lei Bingfu, Deng Jiankun, Zhang Jinyuan, Yan Siyuan, Kuang Haibin, Zang Jinda (2016) Photoluminescence properties and energy transfer between activators at different crystallographic sites in CO3+ doped Sr2MgAl22O36. Ceram Int 42(15):16659–16665
Ping Wu, Pelton Arthur D (1992) Coupled thermodynamic-phase diagram assessment of the rare earth oxide-aluminium oxide binary systems. J Alloys Compd 179:259–287
Udalov YuP, Rakhmankulov RM, Chemekova TYu, Belousova OL (2003) Crystallization and phase equilibria in the Tb2O3–Ga2O3 system. Glass Phys Chem 29(2):200–201
Han YH, Nagata M, Uekawa N, Kakegawa K (2004) Eutectic Al2O3–GdAlO3 composite consolidated by combined rapid quenching and spark plasma sintering technique. Br Ceram Trans 103(5):219–222
Popova VF, Petrosyan AG, Tugova EA, Romanov DP, Gusarov VV (2009) Y2O3–Ga2O3 phase diagram. Russ J Inorg Chem 54(4):624–629
Adonin SA, Peresypkina EV, Sokolov MN, Korolkov IV, Fedin VP (2014) Polyoxomolybdate-supported bismuth trihalides [Mo8O26(BiX3)2]4− (X = Cl, Br, I): syntheses and study of polymorphism. Inorg Chem 53(13):6886–6892
Atuchin VV, Kaichev VV, Korolkov IV, Saraev AA, Troitskaia IB, Perevalov TV, Gritsenko VA (2014) Electronic structure od noncentrosymmetric α-GeO2 with oxygen vacancy: ab initio calculations and comparison with experiment. J Phys Chem C 118:3644–3650
Vasylechko L, Matkovskii A, Savytskii D, Suchocki A, Wallrafen F (1999) Crystal structure of GdFeO3-type rare earth gallates and aluminates. J Alloys Compd 291:57–65
Liu RS, Shi WC, Cheng YC, Huang CY (1997) Crystal structures and peculiar magnetic properties of α- and γ-Al2O3 powders. Mod Phys Lett B 11(26–27):1169–1174
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 32:751–767
Atuchin VV, Andreeva OP, Korolkov IV, Maximovsky EA, Lim CS (2014) Low-temperature synthesis and structural properties of PbMoO4 nanocrystals. Asian J Chem 26(5):1287–1289
Atuchin VV, Chimitova OD, Gavrilova TA, Molokeev MS, Kim SJ, Surovtsev NV, Bazarov BG (2011) Synthesis, structural and vibrational properties of microcrystalline RbNd(MoO4)2. J Cryst Growth 318:683–686
Atuchin VV, Bazarov BG, Gavrilova TA, Grossman VG, Molokeev MS, Bazarova ZhG (2012) Preparation and structural properties of nonlinear optical borates K1(1−x)Rb2x Al2B2O7, 0 < x<0.75. J Alloys Compd 515:119–122
Lim CS, Aleksandrovsky A, Molokeev M, Oreshonkov A, Atuchin V (2015) Microwave sol–gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4:Er3+/Yb3+ phosphors with incommensurately modulated structure. J Solid State Chem 228:160–166
Blasse G, Bril A (1967) Investigation of some Ce3+ activated phosphors. J Chem Phys 47(12):5139–5145
Zych E, Brecher C, Wojtowicz AJ, Lingertat H (1997) Luminescence properties of Ce-activated YAG optical ceramic scintillator materials. J Lumin 75:193–203
Tomiki T, Akamine H, Gishiken M, Kinjoh Y, Miyazato M, Miyazato N, Hiraoka M, Hirata N, Ganahaand Y, Futemma T (1991) Ce3+ centers in YAG single crystals. J Phys Soc Jpn 60(7):2437–2445
Dorenbos Peter (2013) Electronic structure and optical properties of the lanthanide activated RE3(Al1−xGax)5O12 (RE = Gd, Y, Lu) garnet compounds. J Lumin 134:310–318
Acknowledgements
The reported study was funded by RFBR, according to the research projects 16-52-48010 and 17-52-53031, and by the National Natural Science Foundations of China (Grant No. 51511130035). The microstructure observation was performed using the equipment of CKP Nanostructures with the support of RSF (14-22-00143).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galashov, E.N., Atuchin, V.V., Gavrilova, T.A. et al. Synthesis of Y3Al5O12:Ce3+ phosphor in the Y2O3–Al metal–CeO2 ternary system. J Mater Sci 52, 13033–13039 (2017). https://doi.org/10.1007/s10853-017-1427-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1427-5