Abstract
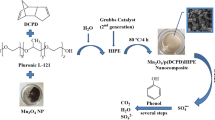
The nZVI@mesoSiO2 nanocomposites with ordered mesoporous structure were successfully prepared through a novel two-step method. Influences of the different conditions on the physicochemical properties and catalytic behavior were investigated. Degradation of 2,4,6-trichlorophenol (2,4,6-TCP) under different conditions was evaluated, and the possible activation mechanism was speculated. The results indicate that only the definite increase in the molar ratio of CTAB/NaOH (cetyltrimethylammonium bromide/sodium hydroxide) helps to obtain the higher specific surface area and narrower pore size distribution of the nZVI@mesoSiO2. The thickness of this silica coating could be easily regulated by changing the dosage of the TEOS (tetraethylorthosilicate) precursor. High dosage of the TEOS (larger than 5 mL) leads to the formation of core-free silica particles and low catalytic activity, while nZVI@mesoSiO2 cannot fully develop at a low dosage (2.5 mL). By controlling different extraction conditions, the nanoscale zero-valent iron core (nZVI) in the composite can be well preserved and corrosion resistant in the whole synthesis process. In this case, the as-prepared nanocomposites offer a high surface area of 638.78 m2 g−1 as well as a large accessible pore volume of 0.49 cm3 g−1 for the degradation, which contributing to a fast and efficient degradation of 2,4,6-TCP from aqueous solutions at an appropriate pH (pH 5.0). Moreover, the reusability investigation indicated that the nZVI cores will be inactivated and the pore will be blocked after repeated cyclic reactions. This comprehensive information provides not only a great understanding of the regulation mechanism of core–shell mesoporous nanocomposites, but also a further useful modification of the composites with high selectivity for 2,4,6-TCP.




















Similar content being viewed by others
References
Lee HY, Lee CL, Jou CJG (2010) Comparison degradation of pentachlorophenol using microwave-induced nanoscale Fe0 and activated carbon. Water Air Soil Pollut 211:17–24. https://doi.org/10.1007/s11270-009-0276-5
Ghosh A, Dutta S, Mukherjee I et al (2017) Template-free synthesis of flower-shaped zero-valent iron nanoparticle: role of hydroxyl group in controlling morphology and nitrate reduction. Adv Powder Technol 28:2256–2264. https://doi.org/10.1016/j.apt.2017.06.006
Zhuang Y, Ahn S, Luthy RG (2010) Debromination of polybrominated diphenyl ethers by nanoscale zerovalent iron: pathways, kinetics, and reactivity. Environ Sci Technol 44:8236–8242. https://doi.org/10.1021/es101601s
Nairat M, Shahwan T, Eroğlu AE, Fuchs H (2015) Incorporation of iron nanoparticles into clinoptilolite and its application for the removal of cationic and anionic dyes. J Ind Eng Chem 21:1143–1151. https://doi.org/10.1016/j.jiec.2014.05.027
Raman CD, Kanmani S (2016) Textile dye degradation using nano zero valent iron: a review. J Environ Manag 177:341–355. https://doi.org/10.1016/j.jenvman.2016.04.034
Fang Z, Qiu X, Huang R et al (2011) Removal of chromium in electroplating wastewater by nanoscale zero-valent metal with synergistic effect of reduction and immobilization. Desalination 280:224–231. https://doi.org/10.1016/j.desal.2011.07.011
Volpe A, Pagano M, Mascolo G et al (2013) Simultaneous Cr(VI) reduction and non-ionic surfactant oxidation by peroxymonosulphate and iron powder. Chemosphere 91:1250–1256. https://doi.org/10.1016/j.chemosphere.2013.02.012
Azzam AM, El-Wakeel ST, Mostafa BB, El-Shahat MF (2016) Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J Environ Chem Eng 4:2196–2206. https://doi.org/10.1016/j.jece.2016.03.048
Guan X, Sun Y, Qin H et al (2015) The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994–2014). Water Res 75:224–248. https://doi.org/10.1016/j.watres.2015.02.034
Noubactep C (2008) A critical review on the process of contaminant removal in Fe0–H2O systems. Environ Technol 29:909–920. https://doi.org/10.1080/09593330802131602
Tang S, Wang XM, Mao YQ et al (2015) Effect of dissolved oxygen concentration on iron efficiency: removal of three chloroacetic acids. Water Res 73:342–352. https://doi.org/10.1016/j.watres.2015.01.027
Gong J, Lee CS, Kim EJ et al (2016) Enhancing the reactivity of bimetallic Bi/Fe0 by citric acid for remediation of polluted water. J Hazard Mater 310:135–142. https://doi.org/10.1016/j.jhazmat.2016.02.027
Gao Y, Wang F, Wu Y et al (2016) Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles. Chem Eng J 285:459–466. https://doi.org/10.1016/j.cej.2015.09.078
Zhuang Y, Jin L, Luthy RG (2012) Kinetics and pathways for the debromination of polybrominated diphenyl ethers by bimetallic and nanoscale zerovalent iron: effects of particle properties and catalyst. Chemosphere 89:426–432. https://doi.org/10.1016/j.chemosphere.2012.05.078
Zhu F, Li L, Ma S, Shang Z (2016) Effect factors, kinetics and thermodynamics of remediation in the chromium contaminated soils by nanoscale zero valent Fe/Cu bimetallic particles. Chem Eng J 302:663–669. https://doi.org/10.1016/j.cej.2016.05.072
Yang Y, Cheng P, Huang S (2016) Unraveling the roles of iron in stabilizing the defective graphene-supported [Formula presented] bimetallic nanoparticles. J Alloys Compd 688:1172–1180. https://doi.org/10.1016/j.jallcom.2016.07.157
Liu J, Liu A, Zhang W (2016) The influence of polyelectrolyte modification on nanoscale zero-valent iron (nZVI): aggregation, sedimentation, and reactivity with Ni(II) in water. Chem Eng J 303:268–274. https://doi.org/10.1016/j.cej.2016.05.132
Phenrat T, Kumloet I (2016) Electromagnetic induction of nanoscale zerovalent iron particles accelerates the degradation of chlorinated dense non-aqueous phase liquid: proof of concept. Water Res 107:19–28. https://doi.org/10.1016/j.watres.2016.10.035
Zhang D, Wei S, Kaila C et al (2010) Carbon-stabilized iron nanoparticles for environmental remediation. Nanoscale 2:917. https://doi.org/10.1039/c0nr00065e
Shi L, Zhang X, Chen Z (2011) Removal of Chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45:886–892. https://doi.org/10.1016/j.watres.2010.09.025
Li A, Tai C, Zhao Z et al (2007) Debromination of decabrominated diphenyl ether by resin-bound iron nanoparticles. Environ Sci Technol 41:6841–6846. https://doi.org/10.1021/es070769c
Mackenzie K, Bleyl S, Georgi A, Kopinke FD (2012) Carbo-Iron—an Fe/AC composite—as alternative to nano-iron for groundwater treatment. Water Res 46:3817–3826. https://doi.org/10.1016/j.watres.2012.04.013
Kresge CT, Leonowicz ME, Roth WJ et al (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712. https://doi.org/10.1038/359710a0
Di Renzo F, Cambon H, Dutartre R (1997) A 28-year-old synthesis of micelle-templated mesoporous silica. Microporous Mater 10:283–286. https://doi.org/10.1016/S0927-6513(97)00028-X
Wang H, Liu Y, Yao S, Zhu P (2018) Selective recognization of dicyandiamide in bovine milk by mesoporous silica SBA-15 supported dicyandiamide imprinted polymer based on surface molecularly imprinting technique. Food Chem 240:1262–1267. https://doi.org/10.1016/j.foodchem.2017.08.066
Dou J, Sheng Y, Choong C et al (2017) Silica nanowires encapsulated Ru nanoparticles as stable nanocatalysts for selective hydrogenation of CO2 to CO. Appl Catal B Environ 219:580–591. https://doi.org/10.1016/j.apcatb.2017.07.083
Gupta R, Ganesan V (2015) Gold nanoparticles impregnated mesoporous silica spheres for simultaneous and selective determination of uric acid and ascorbic acid. Sens Actuators B Chem 219:139–145. https://doi.org/10.1016/j.snb.2015.05.018
Zhang J, Sun W, Bergman L et al (2012) Magnetic mesoporous silica nanospheres as DNA/drug carrier. Mater Lett 67:379–382. https://doi.org/10.1016/j.matlet.2011.09.086
Liu J, Bu W, Pan L, Shi J (2013) NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew Chemie Int Ed 52:4375–4379. https://doi.org/10.1002/anie.201300183
Wang W, Wen Y, Xu L et al (2014) A selective release system based on dual-drug-loaded mesoporous silica for nanoparticle-assisted combination therapy. Chem A Eur J 20:7796–7802. https://doi.org/10.1002/chem.201402334
Wang W, Chen L, Xu LP et al (2015) A free-blockage controlled release system based on the hydrophobic/hydrophilic conversion of mesoporous silica nanopores. Chem A Eur J 21:2680–2685. https://doi.org/10.1002/chem.201405222
Saikia K, Bhattacharya K, Sen D et al (2019) Solvent evaporation driven entrapment of magnetic nanoparticles in mesoporous frame for designing a highly efficient MRI contrast probe. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2018.09.117
Meng C, Zhikun W, Qiang L et al (2018) Preparation of amino-functionalized Fe3O4@mSiO2 core–shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2017.07.062
Raman NK, Anderson MT, Brinker CJ (1996) Template-based approaches to the preparation of amorphous, nanoporous silicas. Chem Mater 8:1682–1701. https://doi.org/10.1021/cm960138+
Beck JS, Vartuli JC, Roth WJ et al (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843. https://doi.org/10.1021/ja00053a020
Zhao W, Gu J, Zhang L et al (2005) Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J Am Chem Soc 127:8916–8917. https://doi.org/10.1021/ja051113r
Huang YH, Zhang TC (2005) Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+. Water Res 39:1751–1760. https://doi.org/10.1016/j.watres.2005.03.002
Kim S-S, Pauly TR, Pinnavaia TJ (2000) Non-ionic surfactant assembly of ordered, very large pore molecular sieve silicas from water soluble silicates. Chem Commun 123:1661–1662. https://doi.org/10.1039/b002856h
Liu Y, Karkamkar A, Pinnavaia TJ (2001) Redirecting the assembly of hexagonal MCM-41 into cubic MCM-48 from sodium silicate without the use of an organic structure modifier. Chem Commun 18:1822–1823. https://doi.org/10.1039/b103954g
Pang X, Tang F (2005) Morphological control of mesoporous materials using inexpensive silica sources. Microporous Mesoporous Mater 85:1–6. https://doi.org/10.1016/j.micromeso.2005.06.012
Yang H, Coombs N, Ozin G (1997) Morphogenesis of shapes and surface patterns in mesoporous silica. Nature 386:692–695
Tang X, Feng Q, Liu K et al (2018) Fabrication of magnetic Fe3O4/silica nanofiber composites with enhanced Fenton-like catalytic performance for Rhodamine B degradation. J Mater Sci. https://doi.org/10.1007/s10853-017-1490-y
Martínez ML, Falcón H, Beltramone AR, Anunziata OA (2016) Synthesis and characterization of 2D-hexagonal, 3D-hexagonal and cubic mesoporous materials using CTAB and silica gel. Mater Des 104:251–258. https://doi.org/10.1016/j.matdes.2016.05.038
Vojoudi H, Badiei A, Bahar S et al (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J Magn Magn Mater 441:193–203. https://doi.org/10.1016/j.jmmm.2017.05.065
Deng Y, Qi D, Deng C et al (2008) Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J Am Chem Soc 130:28–29. https://doi.org/10.1021/ja0777584
Deng Y, Cai Y, Sun Z et al (2010) Multifunctional mesoporous composite microspheres with well-designed nanostructure: a highly integrated catalyst system. J Am Chem Soc 132:8466–8473. https://doi.org/10.1021/ja1025744
Zhou YY, Li X, Chen Z (2012) Rapid synthesis of well-ordered mesoporous silica from sodium silicate. Powder Technol 226:239–245. https://doi.org/10.1016/j.powtec.2012.04.054
Chen Y, Li Z, Qin J, Chen A (2016) Monodispersed mesoporous silica (mSiO2) spheres as abrasives for improved chemical mechanical planarization performance. J Mater Sci. https://doi.org/10.1007/s10853-016-9882-y
Hommaid O, Hamdo JY (2014) Adsorption of chromium(VI) from an aqueous solution on a Syrian surfactant-modified zeolite. Int J ChemTech Res. https://doi.org/10.1016/j.colsurfa.2008.07.025
Wang D, Duan X, Zhang J et al (2009) Fabrication of superparamagnetic hydroxyapatite with highly ordered three-dimensional pores. J Mater Sci. https://doi.org/10.1007/s10853-009-3555-z
Yue Q, Li J, Luo W et al (2015) An interface coassembly in biliquid phase: toward core–shell magnetic mesoporous silica microspheres with tunable pore size. J Am Chem Soc 137:13282–13289. https://doi.org/10.1021/jacs.5b05619
Ding HL, Zhang YX, Wang S et al (2012) Fe3O4@SiO2 core/shell nanoparticles: the silica coating regulations with a single core for different core sizes and shell thicknesses. Chem Mater 24:4572–4580. https://doi.org/10.1021/cm302828d
Lee DC, Mikulec FV, Pelaez JM et al (2006) Synthesis and magnetic properties of silica-coated FePt nanocrystals. J Phys Chem B 110:11160–11166. https://doi.org/10.1021/jp060974z
Zhang M, Cushing BL, O’Connor CJ (2008) Synthesis and characterization of monodisperse ultra-thin silica-coated magnetic nanoparticles. Nanotechnology. https://doi.org/10.1088/0957-4484/19/8/085601
Yi DK, Lee SS, Papaefthymiou GC, Ying JY (2006) Nanoparticle architectures templated by SiO2/Fe2O3 nanocomposites. Chem Mater 18:614–619. https://doi.org/10.1021/cm0512979
LaMer V, Dinegar R (1950) Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem 72:4847–4854. https://doi.org/10.1021/ja01167a001
Huang Y, Pemberton JE (2010) Synthesis of uniform, spherical sub-100 nm silica particles using a conceptual modification of the classic La Mer model. Colloids Surf A Physicochem Eng Asp 360:175–183. https://doi.org/10.1016/j.colsurfa.2010.02.031
Li W, Yang J, Wu Z et al (2012) A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core–shell structures. J Am Chem Soc 134:11864–11867. https://doi.org/10.1021/ja3037146
Liu F, Tian H, He J (2014) Adsorptive performance and catalytic activity of superparamagnetic Fe3O4@nSiO2@mSiO2 core–shell microspheres towards DDT. J Colloids Interface Sci 419:68–72. https://doi.org/10.1016/j.jcis.2013.12.046
Xun S, Jiang W, Guo T et al (2019) Magnetic mesoporous nanospheres supported phosphomolybdate-based ionic liquid for aerobic oxidative desulfurization of fuel. J Colloids Interface Sci. https://doi.org/10.1016/j.jcis.2018.08.115
Zhang J, Zhai S, Li S et al (2013) Pb(II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem Eng J. https://doi.org/10.1016/j.cej.2012.11.043
Li M, Li X, Qi X et al (2015) Shape-controlled synthesis of magnetic iron Oxide@SiO2–Au@C particles with core–shell nanostructures. Langmuir 31:5190–5197. https://doi.org/10.1021/acs.langmuir.5b00800
Li W, Zhao D (2013) Extension of the Stöber method to construct mesoporous SiO2 and TiO2 shells for uniform multifunctional core-shell structures. Adv Mater 25:142–149
Lv Y, Niu Z, Chen Y, Hu Y (2016) Synthesis of SiO2 coated zero-valent iron/palladium bimetallic nanoparticles and their application in a nano-biological combined system for 2,2′,4,4′-tetrabromodiphenyl ether degradation. RSC Adv 6:20357–20365. https://doi.org/10.1039/c5ra22388a
Zaghouane-Boudiaf H, Boutahala M (2011) Adsorption of 2,4,5-trichlorophenol by organo-montmorillonites from aqueous solutions: kinetics and equilibrium studies. Chem Eng J 170:120–126. https://doi.org/10.1016/j.cej.2011.03.039
Acknowledgements
This work was supported by National Natural Science Foundation of China [Nos. 31570568 and 31670585], Science and Technology Planning Project of Guangzhou City, China [Nos. 201607010079, 201607020007], Science and Technology Planning Project of Guangdong Province, China [Nos. 2016A020221005 and 2017A040405022]. The authors are grateful to all the anonymous reviewers for their insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, M., Ma, Y., Wan, J. et al. Investigation of factors affecting the physicochemical properties and degradation performance of nZVI@mesoSiO2 nanocomposites. J Mater Sci 54, 7483–7502 (2019). https://doi.org/10.1007/s10853-018-03312-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-03312-8