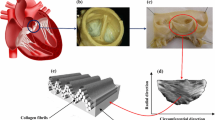
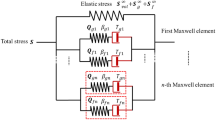
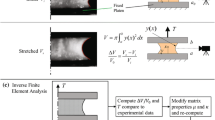
Abstract
The quasi-static mechanical behaviour of the aortic valve (AV) is highly non-linear and anisotropic in nature and reflects the complex collagen fibre kinematics in response to applied loading. However, little is known about the viscoelastic behaviour of the AV. The aim of this study was to investigate porcine AV tissue under uniaxial tensile deformation, in order to establish the directional dependence of its viscoelastic behaviour. Rate dependency associated with different mechanical properties was investigated, and a new viscoelastic model incorporating rate effects developed, based on the Kelvin-Voigt model. Even at low applied loads, experimental results showed rate dependency in the stress–strain response, and also hysteresis and dissipation effects. Furthermore, corresponding values of each parameter depended on the loading direction. The model successfully predicted the experimental data and indicated a ‘shear-thinning’ behaviour. By extrapolating the experimental data to that at physiological strain rates, the model predicts viscous damping coefficients of 8.3 MPa s and 3.9 MPa s, in circumferential and radial directions, respectively. This implies that the native AV offers minimal resistance to internal shear forces induced by blood flow, a potentially critical design feature for substitute implants. These data suggest that the mechanical behaviour of the AV cannot be thoroughly characterised by elastic deformation and fibre recruitment assumptions alone.





Similar content being viewed by others
References
Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129:757–66.
Brewer RJ, Mentzer RM, Deck JD, Ritter RC, Trefil JS, Nolan SP. An in vivo study of the dimensional changes of the aortic valve leaflets during the cardiac cycle. J Thorac Cardiovasc Surg. 1977;74:645–50.
Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—part I: experimental results. J Biomech Eng. 2000;122:23–30.
Merrymanm WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290:H224–31.
Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair: from structure to function: part I. Circulation. 2004;109:942–50.
Vesely I. Heart valve tissue engineering. Circ Res. 2005;97:743–55.
Schoen FJ, Levy RJ. Calcification of tissue heart valve substitute: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80.
Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215.
De Hart J. Fluid-structure interaction in the aortic heart valve. Ph.D. thesis. 2002; Eindhoven University of Technology, Eindhoven, Netherlands.
Schoen FJ, Levy RJ. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47:439–65.
Jamieson WR. Modern cardiac valve devices-bioprostheses and mechanical prostheses: state of the art. J Cardiac Surg. 1993;8:89–98.
Schoen FJ, Levy RJ, Piehler HR. Pathological considerations in replacement cardiac valves. Cardiovasc Pathol. 1992;1:29–52.
Sauren AAHJ, Van Hout MC, Van Steenhoven AA, Veldpaus FE, Janssen JD. The mechanical properties of porcine aortic valve tissues. J Biomech. 1983;16:327–37.
Mavrilas D, Missirlis Y. An approach to the optimization of preparation of bioprosthetic heart valves. J Biomech. 1991;24:331–9.
Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–23.
Christie GW, Barratt-Boyes BG. Biaxial mechanical properties of explanted aortic allograft leaflets. Ann Thorac Surg. 1995;60:S160–4.
Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp—part II: a structural constitutive model. J Biomech Eng. 2000;122:327–35.
Chong M, Missirlis YF. Aortic valve mechanics part II: a stress analysis of the porcine aortic valve leaflets in diastole. Biomat Med Dev Art Org. 1978;6:225–44.
Stella JA, Liao J, Sacks MS. Time dependent biaxial mechanical behaviour of the aortic heart valve leaflet. J Biomech. 2007;40:3169–77.
Ratcliffe A, Mow VC. Articular cartilage. In: Comper WD, editor. Extracellular matrix, vol. 1. Amsterdam: Harwood Academic Publishers; 1996. p. 268–72.
Brody S, Pandit A. Microarchitectural characterization of the aortic heart valve. In: Hasirci N, Hasirci V, editors. Biomaterials: from molecules to engineered tissues. Kluwer Academic/Plenum Publishers; 2004. p. 167–86.
Talman EA, Boughner DR. Internal shear properties of fresh porcine aortic valve cusps: Implications for normal valve function. J Heart Valve Dis. 1996;5:152–9.
Sacks MS, Merryman WD, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42:1804–24.
Liao J, Yang L, Grashow J, Sacks MS. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng. 2007;129:78–87.
Leeson-Dietrich J, Boughner D, Vesely I. Porcine pulmonary and aortic valves: a comparison of their tensile viscoelastic properties at physiological strain rates. J Heart Valve Dis. 1995;4:88–94.
Love ER. Linear superposition in visco-elasticity and theories of delayed effects. Austral J Phys. 1952;9:1–12.
Drapaca CS, Tenti G, Rohlf K, Sivaloganathan S. A quasi-linear viscoelastic constitutive equation for the brain: application to hydrocephalus. J Elast. 2006;85:65–83.
Pioletti DP, Rakotomanana LR, Benvenuti JF, Leyvraz PF. Viscoelastic constitutive law in large deformations: application to human knee ligaments and tendons. J Biomech. 1998;31:753–7.
Fung YC. Biomechanics: mechanical properties of living tissue. 2nd ed. New York: Springer-Verlag New York Inc.; 1993.
Li J, Luo XY, Kuang ZB. A nonlinear anisotropic model for porcine aortic heart valves. J Biomech. 2001;34:1279–89.
Screen HRC, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Insts Mech Engrs Part H: J Eng Med. 2004;218:109–19.
Anssari-Benam A, Viola G, Korakianitis T. Thermodynamic effects of linear dissipative small deformations. J Therm Anal Calorim. 2010;100:941–7.
Teng Z, Tang D, Zheng J, Woodard PK, Hoffman AH. An experimental study on the ultimate strength of the adventitia and media of human atherosclerotic carotid arteries in circumferential and axial directions. J Biomech. 2009;42:2535–9.
Lanir Y. Constitutive equations for fibrous connective tissues. J Biomech. 1983;16:1–12.
Rousseau EPM, Sauren AAHJ, Van Hout MC, Van Steenhoven AA. Elastic and viscoelastic material behaviour of fresh and glutaraldehyde-treated porcine aortic valve tissue. J Biomech. 1983;16:339–48.
Doehring TC, Carew EO, Vesely I. The effect of strain rate on the viscoelastic response of aortic valve tissue: a direct-fit approach. Ann Biomed Eng. 2004;32:223–32.
Acknowledgments
AAB is funded by a studentship from the UK Engineering & Physical Sciences Research Council (EPSRC). Research was also supported by a Discipline Bridging Initiative (DBI) grant from the EPSRC and Medical Research Council (MRC).
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Let the variables (a 1, a 2, a 3) identify a particle in the original configuration of the continuum, and (x 1, x 2, x 3) be the coordinates of that particle when the body is deformed. The deformation of the body is known if (x 1, x 2, x 3) is a known function of (a 1, a 2, a 3) [29]:
This could mathematically be considered as a transformation from a 1, a 2, a 3 to x 1, x 2, x 3. The transformation is one-to-one, such that is the functions in (11) are single-valued, continuous and have a unique inverse:
for every point in continuum [29].
Consider an infinitesimal line element connecting the point P(a 1, a 2, a 3) to a neighbouring point P′(a 1 + da 1, a 2 + da 2, a 3 + da 3). The length of the line PP′ in the original configuration of the continuum will be:
When P and P′ are deformed to the points Q(x 1, x 2, x 3) and Q′(x 1 + dx 1, x 2 + dx 2, x 3 + dx 3), respectively, the length ds of the new deformed QQ′ line is:
From Eqs. 11 and 12 one can get:
Considering Eq. 15, Eqs. 13 and 14 can now be written as:
where δ ij is the Kronecker delta. Now the following can be written:
The Green strain tensor is defined by the term in parenthesis in the above equation as [29]:
Defining the extension ratios in the principle planar directions of 1 and 2 by λ 1 and λ 2, as the ratio of the final length of the element to its original length, we will have:
Now the components of the Green strain tensor in Eq. 19 for deformation in directions 1 and 2 can be written by the extension ratios in Eq. 20 as:
Taking the time derivative of Eq. 18 gives the rate of deformation tensor in terms of the time rate of Green strain tensor:
where v i is the ith component of the in-plane velocity field, x i , and the rate of deformation tensor would be:
For an arbitrary choice of coordination component, and considering Eq. 22, the above equation becomes:
From Eqs. 21 and 24, the components of the rate of deformation tensor in directions 1 and 2 can be obtained as:
Rights and permissions
About this article
Cite this article
Anssari-Benam, A., Bader, D.L. & Screen, H.R.C. A combined experimental and modelling approach to aortic valve viscoelasticity in tensile deformation. J Mater Sci: Mater Med 22, 253–262 (2011). https://doi.org/10.1007/s10856-010-4210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-010-4210-6