Abstract
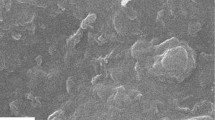
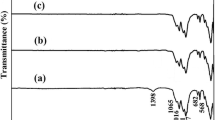
Thick films of calcinated and non calcinated nanobioglass (NBG)-titania composite coatings were prepared on stainless steel substrates by alkoxide sol–gel process. Dip-coating method was used for the films preparation. The morphology, structure and composition of the nano composite films were evaluated using environmental scanning electron microscope, X-ray diffraction and Fourier transform infrared spectroscope. The SEM investigation results showed that prepared thick NBG-titania films are smooth and free of macrocracking, fracture or flaking. The grain size of these films was uniform and nano scale (50–60 nm) which confirmed with TEM. Also FTIR confirmed the presence of Si–O–Si bands on the calcinated NBG-titania films. The hardness of the prepared films (TiO2-calcinated NBG and TiO2-Non calcinated NBG) was compared by using micro hardness test method. The results verified that the presence of calcinated NBG particles in NBG-titania composite enhanced gradually the mechanical data of the prepared films. The in vitro bioactivity of these films was discussed based on the analysis of the variations of Ca and P concentrations in the simulated body fluid (SBF) and their surface morphologies against immersion time. Surface morphology and Si–O–Si bands were found to be of great importance with respect to the bioactivity of the studied films. The results showed that calcinated NBG-titania films have better bioactivity than non calcinated NBG-titania films.












Similar content being viewed by others
References
Hench LL, Spinter RJ, Allen WC, Greenlee TK Jr. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;2(1):117–41.
Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74(1):1487–510.
Williams DF. The biocompatibility and clinical uses of calcium phosphate ceramics. In: Williams DF, editor. Biocompatibility of tissue analogs, vol. 2. Boca Raton: CRC Press; 1985. p. 43–66.
Hulbert SF, Bokros JC, Hench LL, Wilson J, Heimke G. In: Vincenzini P, editor. Ceramics in clinical applications: past, present and future in high tech ceramics. Amsterdam: Elsevier; 1987. p. 189–213.
Le Geros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988;2:164–80.
Yamamuro T, Shikata J, Okumura H, Nakamura T, Yoshii S, Ono K, Kitsugi T. Clinical application of bioactive ceramics in bioceramics. Kitsugi Ishiyaku EuroAmerica, Tokio. 1989;1:175–80.
Cook SD, Thomas KA. Hydroxya apatite-coated metal for orthopaedic and dental implant applications. Abstracts of the 92nd Annual Meeting and Exposition. The American Ceramic Society, Inc., Dallas, TX. 1990.
Yamamuro T, Shikata J, Okumura H, Yoshii S, Konati S, Kokubo T, Heimke G. Pre-clinical and clinical applications of A/W glass–ceramic to various orthopaedic conditions in bioceramics, vol. 2. Cologne: German Ceramic Society; 1990. p. 361–6.
De Groot K. Medical applications of calcium phosphate bioceramics. Centennia l Mem Issue. 1991;0.99(10):943–53.
Hench LL, Wilson JW. Surface-active biomaterials. Science. 1984;226:630–6.
Hench LL. Bioactive glasses and glass–ceramics: a perspective. In: Yamamuro T, Hench LL, Wilson J, editors. Handbook of bioactive ceramics, vol. 1. Boca Raton: CRC Press; 1990. p. 7–23.
Ishizawa H, Fujino M, Ogino M. Surface reactions of calcium phosphate ceramics and glass–ceramics to various physiological solutions. In: Yamamuro T, Hench LL, Wilson J, editors. Handbook of bioactive ceramics, vol. 1. Boca Raton: CRC Press; 1990. p. 115–23.
Ohtsuki C, Aoki Y, Kokubo T, Bando Y, Neo M, Yamamuro T, Nakamura T. Characterization of apatite layer formed on bioactive glass–ceramic A–W. Bioceramics. 1992;5:79–83.
Ohtsuki C, Aoki Y, Kokubo T, Bando Y, Neo M, Yamamuro T, Yacamura T. Characterization of apatite layer formed on bioactive glass–ceramic A–W. Bioceramics. 1992;5:87–94.
Ohtsuki C, Kokubo T, Yamamuro T. Mechanism of apatite formation on CaO–SiO2–P2O5 glasses in simulated body fluid. J Non-Cryst Solids. 1992;143:84–92.
Walker M. An Investigation into the bonding mechanism of bioglass, Doctoral Tesis, Universidad de Florida, 1977.
Kokubo T, Hayashi T, Sakka S, Kitsugi T, Yamamuro T. Bonding between bioactive glass, glass–ceramic or ceramics in simulated body fluid. Yogyo-Ky okai-shi. 1987;0.95(8):785–91.
Ebisawa T, Kokubo T, Ohura K, Yamamuro T. Bioactivity of CaO2·SiO2-based glasses: in vitro evaluation. J Mater Sci Mater Med. 1990;1:239–44.
Boyde A, Manconnachie E, Muller-Mai C. Gross U.SEM study of surface alterations of bioactive glass and glass-ceramics in bony implantation bed. Clin Mater. 1990;5:73–88.
De Aza PN, Guitian F, Merlos A, Lora-Tamayo E, De Aza S. Bioceramics-simulated body fluid interfaces: pH and its influence on hydroxyapatite formation. J Mater Sci Mater Med. 1996;7:399–402.
Sepulveda P, Jones JR, Hench LL. Bioactive sol–gel foams for tissue repair. J Biomed Mater Res. 2002;59:340–8.
Yuron C, Lian Z. Effect of thermal treatment on the microstructure and mechanical properties of gel-derived bioglasses. J Mater Chem Phys. 2005;94:283–7.
Li J, Sun Y, Sun X, Qiao J. Mechanical and corrosion-resistance performance of electrodeposited titania–nickel nanocomposite coatings. J Surf Coat Technol. 2005;192:331–5.
Li P, Kangasniemi I, De Groot K. In vitro and in vivo evaluation of bioactivity of gel titania. Bioceramics. 1993;6:41–5.
Haddow DB, James PF, Van Noort R. Characterisation of sol–gel surfaces for biomedical applications. J Mater Sci: Mater Med. 1996;7:255–60.
Haddow DB, Kothari S, James PF, Short RD, Hatton PV, Van Noort R. Synthetic implant surfaces. 1. The formation and characterisation of sol–gel titania films. Biomaterials. 1996;17:501–7.
Tengvall P, Lundström I. Physico–chemical considerations of titanium as a biomaterial. Clin Mater. 1992;9:115–34.
Miyaji F, Zang X, Yao T, Kokubo T, Ohtsuki C, Kitsugi T, Yamamuro T, Nakamura T. Chemical treatment of Ti metal to induce its bioactivity. Bioceramics. 1994;7:119–24.
Eckert KL, Ha SW, Mathey M, Wintermantel E. Ceramic processing of titania for biomedical applications. Bioceramics. 1995;8:447–52.
Yan WQ, Nakamura T, Kobayashi M, Kokubo T, Kim HM, Miyaji F. Bone-bonding behavior of titanium implants prepared via chemical treatments. Bioceramics. 1996;9:305–9.
Brinker CJ, Hurd AJ, Frye GC, Schunk PR, Ashley CS. In: Hench LL, West JK, editors. Sol-gel thin-film formation in chemical processing of advanced materials. New York: Wiley; 1992. p. 395–413.
Sakka S. Preparation and properties of sol-gel coating films. J Sol–Gel Sci Tech. 1994;2:451–5.
Zhong J, Greenspan DC. Processing and properties of sol–gel bioactive glasses. J Biomed Mater Res. 2000;53:694.
Sepulveda P, Jones JR, Hench LL. In vitro disso- lution of melt-derived 45S5 and sol–gel derived 58S bioactive glasses. J Biomed Mater Res. 2002;61:301.
Bielby RC, Christodoulou IS, Pryce RS, Radford WJP, Hench LL, Polak JM. Time- and concentration-dependent effects of dissolution products of 58S sol-gel bioactive glass on proliferation and differentiation of murine and human osteoblasts. Tissue Eng. 2004;10:1018.
Kokubo T, Kushitani H, Sakka S, Kitshugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramics A-W3. J Biomed Mater Res. 1990;28:721.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475.
Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials. 2001;22:1327.
Akin FA, Zreiqat H, Jordan S, et al. Preparation and analysis of macroporous TiO2 on Ti surfaces for bone-tissue implants. J Biomed Mater Res. 2001;57:588.
Yerokhin AL, Nie X, Leyland A, et al. Plasma electrolysis for surface. Surf. Coat Technol. 1999;122:73.
Duran A, Serna C, Fornes V, Fernandez Navarro JMJ. Structural considerations about SiO2 glasses prepared by sol–gel. Non Cryst Solids. 1986;82(1–3):69–77.
Dutoit DCM, Schmeider M, Baiker A. Titania–silica mixed oxides I Influence of sol–gel and drying conditions on structural properties. J Catal. 1995;153(1):165–76.
Zhang M, Shi L, Shuai Y, Zhao Y, Fang J. A novel route to prepare Ph and temperature-sensitive nanogels via a semibatch process. J Colloid Interface Sci. 2009;330(2):113–8.
Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol–gel processing. J Appl Biomater. 1991;2:231–9.
Vallet-Regi M, Izquierdo-Barba I, Salinas AJ. Influence of P2O5 on crystallinity of apatite formed in vitro on surface of bioactive glasses. J Biomed Mater Res. 1999;46:560–5.
Peltola T, Jokinen M, Rahiala H, Levanen E, Rosenholm JB, Kangasniemi I, Yli-Urpo A. Calcium phosphate formation on porous sol–gel derived SiO2 and CaO–P2O5–SiO2 substrates in vitro. J Biomed Mater Res. 1999;44:12–21.
Izquierdo-Barba I, Salinas AJ, Vallet-Regi M. Effect of the continuous solution exchange on in vitro reactivity of a CaO–SiO2 sol–gel glass. J Biomed Mater Res. 2000;51:191–9.
Xynos I, Edgar A, Buttery L, Hench L, Polak J. Gene-expression profiling of human osteoblasts following treatment with the ionic products of bioglass 45S5 dissoultion. J Biomed Mater Res. 2001;55:151.
Orignac X, Vasconcelos HC, Du XM, Almeida RM. Influence of solvent concentration on the microstructure of SiO2–TiO2 sol-gel films. J Sol-Gel Sci Technol. 1997;8:243–8.
Torruellas WE, Weller Brophy LA, Zanoni R, Stegeman GI, Osborne Z, Zelinski BJJ. Thir-harmonic generation measurement of nonlinearities in SiO2–TiO2 sol-gel films. Appl Phys Lett. 1991;58:1128–30.
Atik M, Zarzycki J. Protective TiO2–SiO2 coatings on stainless steel sheets prepared by a dip-coating technique. J Mater Sci Lett. 1994;13:1301–4.
Almeida RM, Christensen EE. Crystallization behavior of SiO2–TiO2 sol-gel thin films. J Sol-Gel Sci Technol. 1997;8:409–13.
Salama SN, El-Batal HA. Microhardness of phosphate glasses. J Non Cryst Solids. 1994;168:179–85.
Coreno J, Rodriguez R, Araiza MA, Castano VM. Growth of calcium phosphate onto coagulated silica prepared by using modified simulated body fluids. J Mater Chem. 1998;8(12):2807–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dadash, M.S., Karbasi, S., Esfahani, M.N. et al. Influence of calcinated and non calcinated nanobioglass particles on hardness and bioactivity of sol–gel-derived TiO2–SiO2 nano composite coatings on stainless steel substrates. J Mater Sci: Mater Med 22, 829–838 (2011). https://doi.org/10.1007/s10856-011-4270-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4270-2