Abstract
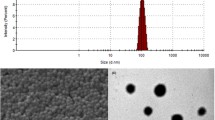
A novel nanoparticles-based brain drug delivery system made of hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) which was surface functionalized with transferrin antibody (OX26) was prepared. Hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) was synthesized, characterized and applied to prepare nanoparticles by means of double emulsion solvent evaporation technique. Transmission electron micrograph and dynamic light scattering showed that nanoparticles had a round and regular shape with a mean diameter of 170 ± 20 nm. Surface chemical composition was detected by X-ray photoelectron spectroscopy. Endomorphins, as a model drug, was encapsulated in the nanoparticles. In vitro drug release study showed that endomorphins was released continuously for 72 h. Cellular uptake study showed that the uptake of nanoparticles by the brain microvascular endothelial cells was both time- and concentration-dependant. Further uptake inhibition study indicated that the uptake of nanoparticles was via a caveolae-mediated endocytic pathway. In vivo endomorphins brain delivery ability was evaluated based upon the rat model of chronic constriction injury of sciatic nerve. OX26 modified nanoparticles had achieved better analgesic effects, compared with other groups. Thus, OX26 modified hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) nanoparticles may be a promising brain drug delivery carrier.












Similar content being viewed by others
References
Begley DJ, Brightman MW. Structural and functional aspects of the blood–brain barrier. Prog Drug Res. 2003;61:39–78.
Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Achim CL, et al. Blood–brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–27.
Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vasc Pharmacol. 2002;38:323–37.
Abbott NJ. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol. 2004;1:407–16.
Teichberg VI. From the liver to the brain across the blood–brain barrier. Proc Natl Acad Sci USA. 2007;104:7315–6.
Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14.
Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J Med Chem. 1980;23:682–4.
Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–8.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53.
Calvo P, Gouritin B, Chacun H, Georgin D, Fattal E, Couvreur P, et al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18:1157–66.
Manoj R, Tracey B, Jennifer L, Ayman EK, Joanne B. Current advances in delivery of biotherapeutics across the blood–brain barrier. Curr Drug Discov Technol. 2011;8:87–101.
Kuo YC, Lin PI, Wang CC. Targeting nevirapine delivery across human brain microvascular endothelial cells using transferrin-grafted poly(lactide-co-glycolide) nanoparticles. Nanomedicine. 2011;6:1011–26.
Huwyler J, Wu DF, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;24:14164–9.
Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14:1–12.
Karatas H, Aktas Y, Bodur E, Yemisci M, Caban S, Vural A, et al. A nanomedicine transports a peptide caspase-3 inhibitor across the blood–brain barrier and provides neuroprotection. J Neurosci. 2009;29:13761–9.
Deeken JF, Loscher W. The blood–brain barrier and cancer: transporters, treatment, and trojan horses. Clin Cancer Res. 2007;13:1663–74.
Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur J Pharm Biopharm. 2009;71:251–6.
Chang J, Jallouli Y, Kroubi M, Yuan XB, Feng W, Betbeder D, et al. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int J Pharmaceut. 2009;379:285–92.
Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)–poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release. 2001;75:211–24.
Li Y, Ogris M, Wagner E, Pelisek J, Ruffer M. Nanoparticles bearing polyethyleneglycol-coupled transferrin as gene carriers: preparation and in vitro evaluation. Int J Pharmaceut. 2003;259:93–101.
Betancourt T, Shah K, Brannon-Peppas L. Rhodamine-loaded poly(lactic-co-glycolic acid) nanoparticles for investigation of in vitro interactions with breast cancer cells. J Mater Sci Mater Med. 2009;20:387–95.
Yang H, Li K, Liu YY, Liu ZH, Miyoshi H. Poly(d,l-lactide-co-glycolide) nanoparticles encapsulated fluorescent isothiocyanate and paclitaxol: preparation, release kinetics and anticancer effect. J Nanosci Nanotechnol. 2009;9:282–7.
Costantino L, Gandolfi F, Bossy-Nobs L, Tosi G, Gurny R, Rivasi F, et al. Nanoparticulate drug carriers based on hybrid poly(d,l-lactide-co-glycolide)-dendron structures. Biomaterials. 2006;27:4635–45.
Sunder A, Kramer M, Hanselmann R, Mulhaupt R, Frey H. Molecular nanocapsules based on amphiphilic hyperbranched polyglycerols. Angew Chem Int Edit. 1999;38:3552–5.
Kainthan RK, Brooks DE. Comparison of hyperbranched and linear polyglycidol unimolecular reverse micelles as nanoreactors and nanocapsules. Macromol Rapid Commun. 2005;26:155–9.
Wilms D, Stiriba SE, Frey H. Hyperbranched polyglycerols: from the controlled synthesis of biocompatible polyether polyols to multipurpose applications. Accounts Chem Res. 2010;43:129–41.
Olivier JC, Huertas R, Lee HJ, Calon F, Pardridge WM. Synthesis of pegylated immunonanoparticles. Pharm Res. 2002;19:1137–43.
Tomboly C, Peter A, Toth G. In vitro quantitative study of the degradation of endomorphins. Peptides. 2002;23:1573–80.
Sunder A, Hanselmann R, Frey H, Mulhaupt R. Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization. Macromolecules. 1999;32:4240–6.
Dehouck MP, Jolliet-Riant P, Bree F, Fruchart JC, Cecchelli R, Tillement JP. Drug transfer across the blood–brain barrier: correlation between in vitro and in vivo models. J Neurochem. 1992;58:1790–7.
Meresse S, Dehouck MP, Delorme P, Bensaid M, Tauber JP, Delbart C, et al. Bovine brain endothelial cells express tight junctions and monoamine oxidase activity in long-term culture. J Neurochem. 1989;53:1363–71.
Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63.
Holter D, Burgath A, Frey H. Degree of branching in hyperbranched polymers. Acta Polym. 1997;48:30–5.
Scholes PD, Coombes AGA, Illum L, Davis SS, Watts JF, Davies MC, et al. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J Control Release. 1999;59:261–78.
Quellec P, Gref R, Dellacherie E, Sommer F, Tran MD, Alonso MJ. Protein encapsulation within poly(ethylene glycol)-coated nanospheres. II. Controlled release properties. J Biomed Mater Res. 1999;47:388–95.
Gan CW, Feng SS. Transferrin-conjugated nanoparticles of poly(lactide)-d-α-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood–brain barrier. Biomaterials. 2010;31:7748–57.
Baratchi S, Kanwar RK, Ashok C, Hittu M, Parratt A, Sahoo SK, et al. Promises of nanotechnology for drug delivery to brain in neurodegenerative diseases. Curr Nanosci. 2009;5:15–25.
Prabha S, Labhasetwar V. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm Res. 2004;21:354–64.
Discher BM, Won YY, Ege DS, Bates FS, Discher DE, Hammer DA, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284:1143–6.
Thole M, Nobmann S, Huwyler J, Bartmann A, Fricker G. Uptake of cationized albumin coupled liposomes by cultured porcine brain microvessel endothelial cells and intact brain capillaries. J Drug Target. 2002;10:337–44.
Schroder U, Sabel BA. Nanoparticles, a drug carrier system to pass the blood–brain barrier, permit central analgesic effects of i.v. dalargin injections. Brain Res. 1996;710:121–4.
Das D, Lin S. Double-coated poly(butylcynanoacrylate) nanoparticulate delivery systems for brain targeting of dalargin via oral administration. J Pharm Sci. 2005;94:1343–53.
Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995;674:171–4.
Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099–106.
Acknowledgments
This study was financially supported by China National Natural Scientific Found (30700780), Beijing Natural Scientific Found (7102052) and Beijing Nova Program (2008A083).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, H., Jin, X., Li, L. et al. OX26 modified hyperbranched polyglycerol-conjugated poly(lactic-co-glycolic acid) nanoparticles: synthesis, characterization and evaluation of its brain delivery ability. J Mater Sci: Mater Med 23, 1891–1901 (2012). https://doi.org/10.1007/s10856-012-4658-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4658-7