Abstract
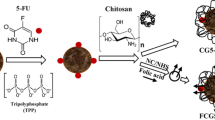
Chitosan (CS) was first modified hydrophobically with deoxycholic acid (DCA) and then with polyethylene glycol (PEG) to obtain a novel amphiphilic polymer (CS–DCA–PEG). This was covalently bound to folic acid (FA) to develop nanoparticles (CS–DCA–PEG–FA) with tumor cell targeting property. The structure of the conjugates was characterised using Fourier transform infrared and 1H nuclear magnetic resonance spectroscopy and X-ray diffraction. Based on self-aggregation, the conjugates formed nanoparticles with a low critical aggregation concentration of 0.035 mg/ml. The anti-cancer drug doxorubicin (DOX) was encapsulated into the nanoparticles with a drug-loading capacity of 30.2 wt%. The mean diameter of the DOX-loaded nanoparticles was about 200 nm, with a narrow size distribution. Transmission electron microscopy images showed that the DOX-loaded nanoparticles were spherical. The drug release was studied under different conditions. Furthermore, the cytotoxic activities of DOX in CS–DCA–PEG–FA nanoparticles against folate receptor (FR)-positive HeLa cells and FR-negative fibroblast 3T3 cells were evaluated. These results suggested that the CS–DCA–PEG–FA nanoparticles may be a promising vehicle for the targeting anticancer drug to tumor cells.









Similar content being viewed by others
References
Sahu SK, Maiti S, Maiti TK, Ghosh SK, Pramanik P. Hydrophobically modified carboxymethyl chitosan nanoparticles targeted delivery of paclitaxel. J Drug Target. 2011;19:104–13.
Wang FH, Zhang DR, Duan CX, et al. Preparation and characterizations of a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid conjugates and self-aggregates. Carbohyd Polym. 2011;84:1192–200.
Ohya Y, Takeda S, Shibata Y, Ouchi T, Maruyama A. Preparation of highly stable biodegradable polymer micelles by coating with polyion complex. Macromol Chem Phys. 2010;211:1750–6.
Huo MR, Zhang Y, Zhou JP, et al. Synthesis and characterization of low-toxic amphiphilic chitosan derivatives and their application as micelle carrier for antitumor drug. Int J Pharmaceut. 2010;394:162–73.
Li PW, Wang YC, Zeng FB, Chen LJ, Peng Z, Kong LX. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohyd Res. 2011;346:801–6.
Zhou HF, Yu WT, Guo X, et al. Synthesis and characterization of amphiphilic glycidol-chitosan-deoxycholic acid nanoparticles as a drug carrier for doxorubicin. Biomacromolecules. 2010;11:3480–6.
Jin YH, Hu HY, Qiao MX, et al. pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: preparation and in vitro evaluation. Colloid Surface B. 2012;94:184–91.
Cao MY, Jin HX, Ye WJ, Liu P, Wang LQ, Jiang HL. A convenient scheme for synthesizing reduction-sensitive chitosan-based amphiphilic copolymers for drug delivery. J Appl Polym Sci. 2012;123:3137–44.
Wang BQ, He CB, Tang C, Yin CH. Effects of hydrophobic and hydrophilic modifications on gene delivery of amphiphilic chitosan based nanocarriers. Biomaterials. 2011;32:4630–8.
Wang J, Zong JY, Zhao D, Zhuo RX, Cheng SX. In situ formation of chitosan–cyclodextrin nanospheres for drug delivery. Colloid Surface B. 2011;87:198–202.
Zeng P, Xu Y, Zeng CH, Ren H, Peng ML. Chitosan-modified poly(d,l-lactide-co-glycolide) nanospheres for plasmid DNA delivery and HBV gene-silencing. Int J Pharmaceut. 2011;415:259–66.
Huynh DP, Nguyen MK, Pi BS, et al. Functionalized injectable hydrogels for controlled insulin delivery. Biomaterials. 2008;29:2527–34.
Gao JQ, Zhao QQ, Lv TF, et al. Gene-carried chitosan-linked-PEI induced high gene transfection efficiency with low toxicity and significant tumor-suppressive activity. Int J Pharmaceut. 2010;387:286–94.
Huh MS, Lee SY, Park S, et al. Tumor-homing glycol chitosan/polyethylenimine nanoparticles for the systemic delivery of siRNA in tumor-bearing mice. J Control Release. 2010;144:134–43.
Duan CX, Zhang DR, Wang FH, et al. Chitosan-g-poly(N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int J Pharmaceut. 2011;409:252–9.
Cai GQ, Jiang HL. pH-sensitive nanoparticles self-assembled from a novel class of biodegradable amphiphilic copolymers based on chitosan. J Mater Sci-Mater M. 2009;20:1315–20.
Yang SJ, Chang SM, Tsai KC, Tsai HM, Chen WS, Shieh MJ. Enhancement of chitosan nanoparticle-facilitated gene transfection by ultrasound both in vitro and in vivo. J Biomed Mater Res B. 2012;100B:1746–54.
Wang XH, Tian Q, Wang W, Zhang CN, Wang P, Yuan Z. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. J Mater Sci-Mater M. 2012;23:1663–74.
Du J, Zhang S, Sun R, Zhang LF, Xiong CD, Peng YX. Novel polyelectrolyte carboxymethyl konjac glucomannan–chitosan nanoparticles for drug delivery. II. Release of albumin in vitro. J Biomed Mater Res B. 2005;72B:299–304.
Duan JH, Liu MJ, Zhang YD, Zhao JF, Pan YF, Yang XY. Folate-decorated chitosan/doxorubicin poly(butyl)cyanoacrylate nanoparticles for tumor-targeted drug delivery. J Nanopart Res. 2012;14.
Galbiati A, Tabolacci C, DellaRocca BM, et al. Targeting tumor cells through chitosan–folate modified microcapsules loaded with camptothecin. Bioconjugate Chem. 2011;22:1066–72.
Lee KY, Kim JH, Kwon IC, Jeong SY. Self-aggregates of deoxycholic acid modified chitosan as a novel carrier of adriamycin. Colloid Polym Sci. 2000;278:1216–9.
Wang F, Chen Y, Zhang D, et al. Folate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid micelles. Int J Nanomed. 2012;7:325–37.
Wang F, Zhang D, Duan C, et al. Preparation and characterizations of a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid conjugates and self-aggregates. Carbohyd Polym. 2011;84:1192–200.
Lv PP, Ma YF, Yu R, et al. Targeted delivery of insoluble cargo (Paclitaxel) by PEGylated chitosan nanoparticles grafted with Arg-Gly-Asp (RGD). Mol Pharmaceut. 2012;9:1736–47.
Hou ZQ, Zhan CM, Jiang QW, Hu Q, Li L, Chang D, et al. Both FA- and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res Lett. 2011;6.
Oh NM, Oh KT, Baik HJ, et al. A self-organized 3-diethylaminopropyl-bearing glycol chitosan nanogel for tumor acidic pH targeting: in vitro evaluation. Colloid Surface B. 2010;78:120–6.
Wang HJ, Zhao PQ, Liang XF, et al. Folate-PEG coated cationic modified chitosan—cholesterol liposomes for tumor-targeted drug delivery. Biomaterials. 2010;31:4129–38.
Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128:23–31.
Shen JM, Tang WJ, Zhang XL, Chen T, Zhang HX. A novel carboxymethyl chitosan-based folate/Fe3O4/CdTe nanoparticle for targeted drug delivery and cell imaging. Carbohyd Polym. 2012;88:239–49.
Kulkarni AR, Lin YH, Liang HF, Chang WC, Hsiao WW, Sung HW. A novel method for the preparation of nanoaggregates of methoxy polyethyleneglycol linked chitosan. J Nanosci Nanotechnol. 2006;6:2867–73.
Mansouri S, Cuie Y, Winnik F, et al. Characterization of folate–chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27:2060–5.
Ji JG, Wu DJ, Liu L, Chen JD, Xu Y. Preparation, characterization, and in vitro release of folic acid-conjugated chitosan nanoparticles loaded with methotrexate for targeted delivery. Polym Bull. 2012;68:1707–20.
Yue ZG, Wei W, Lv PP, et al. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules. 2011;12:2440–6.
Acknowledgments
This study is financially supported by the Natural Science Foundation of Guangdong (S2012040008003), Guangzhou Science and Technology Plan Project (No. 11C32070752), the Key Project of DEGP (cxzd1109), the Ph.D. Programs Foundation of Ministry of Education of China and the Fundamental Research Funds for the Central Universities (21612327). This research is financially supported by Guangzhou Science and Technology Plan Project (No. 11C32070752).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Z., Guo, R., Li, W. et al. Nanoparticles of deoxycholic acid, polyethylene glycol and folic acid-modified chitosan for targeted delivery of doxorubicin. J Mater Sci: Mater Med 25, 723–731 (2014). https://doi.org/10.1007/s10856-013-5113-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-013-5113-0