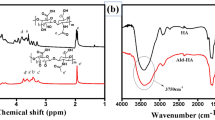
Abstract
Amphiphilic biosynthetic hydrogels comprising natural polysaccharide alginate (I) and synthetic polyester polypropylene fumarate (II) units were prepared by crosslinking the copolymer of I and II with calcium ion and vinyl monomers viz, 2-hydroxyethyl methacrylate (HEMA), methyl methacrylate (MMA), butyl methacrylate (BMA) and N,N′-methylene bisacrylamide (NMBA). Three fast degradable hydrogels, ALPF-MMA, ALPF-HEMA and ALPF-BMA and one slow degradable hydrogel ALPF-NMBA were prepared. These hydrogels are amphiphilic and able to hold sufficient amount of proteins on their surfaces. All these hydrogels are found to be hemocompatible, cytocompatible and genocompatible. ALPF-NMBA promotes infiltration of L929 fibroblasts and 3D growth of H9c2 cardiomyoblasts and long-term viability.










Similar content being viewed by others
References
Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–75.
Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–90.
Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342.
Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation. 1991;84:2123–34.
Holmes JW, Yamashita H, Waldman LK, Covell JW. Scar remodeling and transmural deformation after infarction in the pig. Circulation. 1994;90:411–20.
Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, et al. Challenges in cardiac tissue engineering. Tissue Eng B. 2010;16:169–87.
Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144–9.
Wan WK, Campbell G, Zhang ZF, Hui AJ, Boughner DR. Optimising the tensile properties of poly vinyl alcohol hydrogel for the construction of bioporsthetic heart valve stent. J Biomed Mater Res. 2002;63:854–61.
Dar A, Shachar M, Leor J, Cohen S. Optimisation of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80:305–12.
Chandy T, Rao GH, Wilson RF, Das GS. The development of porous alginate/elastin/PEG composite matrix for cardiovascular engineering. J Biomater Appl. 2003;17:287–301.
Cramer JW, Ginde S, Bartz PJ, Tweddell JS, Litwin SB, Earing MG. Aortic aneurysms remain a significant source of morbidity and mortality after use of Dacron @ patch aortoplasty to repair coarctation of the aorta: results from a single center. Pediatr Cardiol. 2013;34:296–301.
Kelly DJ, Rosen AB, Schuldt AJT, Kochupura PV, Doronin SV, Potapova IA, et al. Increased myocyte content and mechanical function within a tissue-engineered myocardial patch following implantation. Tissue Eng Part A. 2009;15:2189–201.
Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg. 2007;83:648–54.
Wainwright JM, Hashizume R, Fujimoto KL, Remlinger NT, Pesyna C, Wagner WR, Tobita K, Gilbert TW, Badylak SF. Right ventricular outflow tract repair with cardiac biologic scaffold. Cells Tissues Organs. 2012;195:159–70.
Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009. doi:10.1073/pnas.0908381106.
Dausse Y, Grossin L, Miralles G, Pelletier S, Mainard D, Hubert P, et al. Cartilage repair using new polysaccharidic biomaterials: macroscopic, histological and biochemical approaches in a rat model of cartilage defect. Osteoarthritis Cartilage. 2003;11:16–28.
Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–408.
Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–51.
Jayabalan M, Shalumon KT, Mitha MK. Injectable biomaterials for minimally invasive orthopedic treatments. J Mater Sci Mater Med. 2009;20:1379–87.
Mitha MK, Jayabalan M. Studies on biodegradable and crosslinkable poly(castor oil fumarate)/poly(propylene fumarate) composite adhesive as a potential injectable biomaterial. J Mater Sci Mater Med. 2009;20(Suppl 1):S203–11.
Gnanaprakasam Thankam F, Muthu J, Sankar V, Kozhiparambil Gopal R. Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloids Surf. B Biointerfaces. 2013;107:137–45.
Gnanaprakasam Thankam F, Muthu J. Influence of plasma protein–hydrogel interaction moderated by absorption of water on long-term cell viability in amphiphilic biosynthetic hydrogels. RSC Adv. 2013;3:24509.
Thankam Finosh G, Jayabalan M. Reactive oxygen species—Control and management using amphiphilic biosynthetic hydrogels for cardiac applications. Adv. Biosci. Biotechnol. 2013;04:1134–46.
Thankam FG, Muthu J. Biosynthetic hydrogels-studies on chemical and physical characteristics on long-term cellular response for tissue engineering: biosynthetic hydrogels. J Biomed Mater Res A. 2013. doi:10.1002/jbm.a.34895.
Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2010;43:581–91.
Lee W, Cho N-J, Xiong A, Glenn JS, Frank CW. Hydrophobic nanoparticles improve permeability of cell-encapsulating poly(ethylene glycol) hydrogels while maintaining patternability. Proc Natl Acad Sci USA. 2010;107:20709–14.
Vogler EA. Water and the acute biological response to surfaces. J Biomater Sci Polym Ed. 1999;10:1015–45.
Nuttelman CR, Henry SM, Anseth KS. Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials. 2002;23:3617–26.
Hunt NC, Smith AM, Gbureck U, Shelton RM, Grover LM. Encapsulation of fibroblasts causes accelerated alginate hydrogel degradation. Acta Biomater. 2010;6:3649–56.
Cao L, Xie L, Xue X, Tan H, Liu Y, Zhou S. Purification and characterization of alginate lyase from streptomyces species strain A5 isolated from banana rhizosphere. J Agric Food Chem. 2007;55:5113–7.
Dobrovolskaia MA, Clogston JD, Neun BW, Hall JB, Patri AK, McNeil SE. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008;8:2180–7.
Neu B, Meiselman HJ. Depletion-mediated red blood cell aggregation in polymer solutions. Biophys J. 2002;83:2482–90.
Rossi NAA, Mustafa I, Jackson JK, Burt HM, Horte SA, Scott MD, et al. In vitro chelating, cytotoxicity, and blood compatibility of degradable poly(ethylene glycol)-based macromolecular iron chelators. Biomaterials. 2009;30:638–48.
Lee JH, Khang G, Lee JW, Lee HB. Platelet adhesion onto chargeable functional group gradient surfaces. J Biomed Mater Res. 1998;40:180–6.
Qu X-H, Wu Q, Chen G-Q. In vitro study on hemocompatibility and cytocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J Biomater Sci Polym Ed. 2006;17:1107–21.
Schulte VA, Diez M, Möller M, Lensen MC. Topography-induced cell adhesion to Acr-sP(EO-stat-PO) hydrogels: the role of protein adsorption. Macromol Biosci. 2011;11:1378–86.
Sweryda-Krawiec B, Devaraj H, Jacob G, Hickman JJ. A new interpretation of serum albumin surface passivation. Langmuir. 2004;20:2054–6.
Van Tienhoven EAE, Korbee D, Schipper L, Verharen HW, De Jong WH. In vitro and in vivo (cyto)toxicity assays using PVC and LDPE as model materials. J Biomed Mater Res A. 2006;78:175–82.
Baskić D, Popović S, Ristić P, Arsenijević NN. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int. 2006;30:924–32.
Widziewicz K, Kalka J, Skonieczna M, Madej P. The comet assay for the evaluation of genotoxic potential of landfill leachate. Sci World J. 2012;2012:1–8.
Chauvel-Lebret DJ, Auroy P, Tricot-Doleux S, Bonnaure-Mallet M. Evaluation of the capacity of the SCGE assay to assess the genotoxicity of biomaterials. Biomaterials. 2001;22:1795–801.
Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101.
Deten A, Volz HC, Briest W, Zimmer H-G. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–40.
Creemers EEJM, Cleutjens JPM, Smits JFM, Daemen MJAP. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–10.
Shiomi T, Tsutsui H, Hayashidani S, Suematsu N, Ikeuchi M, Wen J, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106:3126–32.
Segers VFM, Lee RT. Protein therapeutics for cardiac regeneration after myocardial infarction. J Cardiovasc Transl Res. 2010;3:469–77.
Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor beta1 released from a biodegradable hydrogel. J Control Release. 2000;64:133–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thankam, F.G., Muthu, J. Infiltration and sustenance of viability of cells by amphiphilic biosynthetic biodegradable hydrogels. J Mater Sci: Mater Med 25, 1953–1965 (2014). https://doi.org/10.1007/s10856-014-5234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5234-0