Abstract
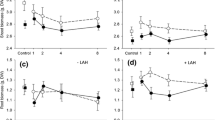
Herbivore-induced plant responses can significantly change as a function of plant developmental stage and previous history of damage. Yet, empirical tests that assess the combined role of multiple damage events and age-dependent constraints on the ability of plants to induce defenses within and among tissues are scarce. This question is of particular interest for annual and/or short-lived perennial plant species, whose responses to single or multiple damage events over a growing season are likely to interact with ontogenetic constraints in affecting a plant’s ability to respond to herbivory. Using Plantago lanceolata and one of its specialist herbivores, Junonia coenia, we examined the effect of plant ontogeny (juvenile vs. mature developmental stages) and history of damage (single and multiple damage events early and/or late in the season) on plant responses to leaf damage. Plant responses to herbivory were assessed as induced chemical defenses (iridoid glycosides) and compensatory regrowth, in both above- and below-ground tissues. We found that constitutive concentration of iridoid glycosides markedly increased as plants matured, but plant ability to induce chemical defenses was limited to juvenile, but not mature, plant stages. In addition, induced defenses observed 7 d following herbivory in juvenile plants disappeared 5 wk after the first herbivory event, and mature plants that varied considerably in the frequency and intensity of damage received over 5 wk, did not differ significantly in their levels of chemical defenses. Also, only small changes in compensatory regrowth were detected. Finally, we did not observe changes in below-ground tissues’ defenses or biomass a week following 50% removal of leaf tissues at either age class or history of damage. Together, these results suggest that in P. lanceolata and perhaps other systems, ontogenetic trajectories in plant growth and defenses leading to strong age-dependent induced responses may prevail over herbivore-induced indirect interactions.


Similar content being viewed by others
References
Adler, L. S., Schmitt, J., and Bowers, M. D. 1995. Genetic variation in defensive chemistry in Plantago lanceolata (Plantaginaceae) and its effect on the specialist herbivore Junonia coenia (Nymphalidae). Oecologia 101:75–85.
Baldwin, I. T. and Schmelz, E. A. 1996. Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology 77:236–246.
Barton, K. E. 2007. Early ontogenetic patterns in chemical defense in Plantago (Plantaginaceae): Genetic variation and trade-offs. Am. J. Bot. 94:56–66.
Barton, K. E. 2008. Phenotypic plasticity in seedling defense strategies: compensatory regrowth and chemical induction. Oikos 117:917–925.
Barton, K. E. and Koricheva, J. 2010. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am. Nat. 175:481–493.
Bellostas, N., Sorensen, J. C., and Sorensen, H. 2007. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 87:1587–1594.
Beninger, C. W., Cloutier, R. R., Monteiro, M. A., and Grodzinski, B. 2007. The distribution of two major iridoids in different organs of Antirrhinum majus L. at selected stages of development. J. Chem. Ecol. 33:731–747.
Biere, A., Marak, H. B., and Van Damme, J. M. M. 2004. Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia 140:430–441.
Boege, K. and Marquis, R. J. 2005. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol. Evol. 20:441–448.
Boege, K., Dirzo, R., Siemens, D., and Brown, P. 2007. Ontogenetic switches from plant resistance to tolerance: minimizing costs with age? Ecol. Lett. 10:177–187.
Bowers, M. D. 1983. Iridoid glycosides and larval hostplant specificity in checkerspot butterflies (Euphydryas, Nymphalidae). J. Chem. Ecol. 9:475–493.
Bowers, M. D. 1984. Iridoid glycosides and host-plant specificity in larvae of the Buckeye butterfly, Junonia coenia (Nymphalidae). J. Chem. Ecol. 10:1567–1577.
Bowers, M. D. 1991. Iridoid glycosides, pp 297–326, in G. A. Rosenthal and M. R. Berenbaum (eds), Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press, Inc., San Diego, California, USA.
Bowers, M. D. and Puttick, G. M. 1989. Iridoid glycosides and insect feeding preferences - Gypsy moths (Lymantria dispar, Lymantriidae) and Buckeyes (Junonia coenia, Nymphalidae). Ecol. Entomol. 14:247–256.
Bowers, M. D. and Stamp, N. E. 1993. Effects of plant age, genotype, and herbivory on Plantago performance and chemistry. Ecology 74:1778–1791.
Camara, M. D. 1997. Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hubner (Nymphalidae): A gravimetric and quantitative genetic analysis. Evol. Ecol. 11:451–469.
Cavers, P. B., Bassett, I. J., and Crompton, C. W. 1980. The biology of Canadian weeds. 47. Plantago lanceolata L. Can. J. Plant Sci. 60:1269–1282.
Cipollini, D. F., Purrington, C. B., and Bergelson, J. 2003. Cost of induced responses in plants. Bas. Appl. Ecol. 4:79–85.
Darrow, K. and Bowers, M. D. 1999. Effects of herbivore damage and nutrient level on induction of iridoid glycosides in Plantago lanceolata. J. Chem. Ecol. 25:1427–1440.
de Deyn, G. B., Raaijmakers, C. E., van Ruijven, J., Berendse, F., and van der Putten, W. H. 2004. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106:576–586.
Dyer, L. A., and Bowers, M. D. 1996. The importance of sequestered iridoid glycosides as a defense against an ant predator. J. Chem. Ecol. 22:1527–1539.
Erb, M., Robert, C. A. M., Hibbard, B. E., and Turlings, T. C. J. 2011. Sequence of arrival determines plant-mediated interactions between herbivores. J. Ecol. 99:7–15.
Fuchs, A. and Bowers, M. D. 2004. Patterns of iridoid glycoside production and induction in Plantago lanceolata and the importance of plant age. J. Chem. Ecol. 30:1723–1741.
Harvey, J. A., Van Nouhuys, S., and Biere, A. 2005. Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J. Chem. Ecol. 31:287–302.
Haukioja, E. and Koricheva, J. 2000. Tolerance to herbivory in woody vs. herbaceous plants. Evol. Ecol. 14:551–562.
Hogedal, B. D. and Molgaard, P. 2000. HPLC analysis of the seasonal and diurnal variation of iridoids in cultivars of Antirrhinum majus. Biochem. Syst. Ecol. 28:949–962.
Jamieson, M. A. and Bowers, M. D. 2010. Iridoid glycoside variation in the invasive plant Dalmatian Toadflax, Linaria dalmatica (Plantaginaceae), and sequestration by the biological control agent, Calophasia lunula. J. Chem. Ecol. 36:70–79.
Jarzomski, C. M., Stamp, N. E., and Bowers, M. D. 2000. Effects of plant phenology, nutrients and herbivory on growth and defensive chemistry of plantain, Plantago lanceolata. Oikos 88:371–379.
Kaplan, I. and Denno, R. F. 2007. Interspecific interactions in phytophagous insects revised: a quantitative assessment of competition theory. Ecol. Lett. 10:977–994.
Kaplan, I., Halitschke, R., Kessler, A., Sardanelli, S., and Denno, R. F. 2008. Constitutive and induced defenses to herbivory on above- and belowground plant tissues. Ecology 89:392–406.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago.
Muola, A., Mutikainen, P., Laukkanen, L., Lilley, M., and Leimu, R. 2010. Genetic variation in herbivore resistance and tolerance: the role of plant life-history stage and type of damage. J. Evol. Biol. 23:2185–2196.
Ohgushi, T. 2005. Indirect interaction webs: Herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 36:81–105.
Ohnmeiss, T. E. and Baldwin, I. T. 2000. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 81:1765–1783.
Orians, C. M., Hochwender, C. G., Fritz, R. S., and Snall, T. 2010. Growth and chemical defense in willow seedlings: trade-offs are transient. Oecologia 163:283–290.
Pereyra, P. and Bowers, M. D. 1988. Iridoid glycosides as oviposition stimulants for the buckeye, Junonia coenia (Nymphalidae). J. Chem. Ecol. 14:917–928.
Poelman, E. H., Broekgaarden, C., Van Loon, J. J. A., and Dicke, M. 2008. Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 17:3352–3365.
Prudic, K. L., Oliver, J. C., and Bowers, M. D. 2005. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143:578–587.
Quintero, C., and Bowers, M. D. 2011. Changes in plant chemical defenses and nutritional quality as a function of ontogeny in Plantago lanceolata (Plantaginaceae). Oecologia (In press).
Roitto, M., Rautio, P., Markkola, A., Julkunen-Tiitto, R., Varama, M., Saravesi, K., and Tuomi, J. 2009. Induced accumulation of phenolics and sawfly performance in Scot pine in response to previous defoliation. Tree Physiol. 29:207–216.
Ronsted, N., Gobel, E., Franzyk, H., Jensen, S. R., and Olsen, C. E. 2000. Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. Phytochemistry 55:337–48.
Ruuhola, T., Salminen, J. P., Haviola, S., Yang, S., and Rantala, M. J. 2007. Immunological memory of Mountain Birches: effects of phenolics on performance of the Autumnal moth depend on herbivory history of trees. J. Chem. Ecol. 33:1160–1176.
Saastamoinen, M., Van Nouhuys, S., Nieminen, M., O’hara, B., and Suomi, J. 2007. Development and survival of a specialist herbivore, Melitaea cinxia, on host plants producing high and low concentrations of iridoid glycosides. Ann Zool Fenn 44:70–80.
Shefferson, R. P. and Roach, D. A. 2010. Longitudinal analysis of Plantago: adaptive benefits of iteroparity in a short-lived, herbaceous perennial. Ecology 91:441–447.
Smilanich, M. A., Dyer, L. A., Chambers, J. Q., and Bowers, M. D. 2009. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol Lett 12:612–621.
Strauss, S. Y. and Agrawal, A. A. 1999. The ecology and evolution of plant tolerance to herbivory. Tr. Ecol. Evol. 14:179–185.
Strohmeyer, H. H., Stamp, N. E., Jarzomski, C. M., and Bowers, M. D. 1998. Prey species and prey diet affect growth of invertebrate predators. Ecol Entomol 23:68–79.
Talsma, J. H. R., Torri, K., and Van Nouhuys, S. 2008. Host plant use by the Heath fritillary butterfly, Melitaea athalia: plant habitat, species and chemistry. Arthropod-Plant Inter. 2:63–75.
Thaler, J. S., Fidantsef, A. L., and Bostock, R. M. 2002. Antagonism between jasmonate- and salicylate-mediated induced plant resistance: Effects of concentration and timing of elicitation on defense-related proteins, herbivore, and pathogen performance in tomato. J. Chem. Ecol. 28:1131–1159.
Theodoratus, D. H. and Bowers, M. D. 1999. Effects of sequestered iridoid glycosides on prey choice of the prairie wolf spider, Lycosa carolinensis. J. Chem. Ecol. 25:283–295.
Thomas, S. C., Sztaba, A. J., and Smith, S. M. 2010. Herbivory patterns in mature sugar maple: variation with vertical canopy strata and tree ontogeny. Ecol. Entomol. 35:1–8.
Tucker, C. and Avila-Sakar, G. 2010. Ontogenetic changes in tolerance to herbivory in Arabidopsis. Oecologia 164:1005–1015.
Van Dam, N. M., Horn, M., Mares, M., and Baldwin, I. T. 2001. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J. Chem. Ecol. 27:547–568.
Van ZANDT, P. A. and AGRAWAL, A. A. 2004. Community-wide impacts of herbivore-induced plant responses in milkweed (Asclepias syriaca). Ecology 85:2616–2629.
Verhage, A., Van Wees, S. C. M., and Pietersen, C. M. J. 2010. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 154:536–540.
Viswanathan, D. V., Lifchits. O. A., and Thaler, J. S. 2007. Consequences of sequential attack for resistance to herbivores when plants have specific induced responses. Oikos 116:1389–1399.
Waltz, A. M. and Whitham, T. G. 1997. Plant development affects arthropod communities: opposing impacts of species removal. Ecology 78:2133–2144.
Warner, P. J. and Cushman, J. H. 2002. Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia 132:77–85.
Williams, R. D. and Ellis, B. E. 1989. Age and tissue distribution of alkaloids in Papaver somniferum. Phytochemistry 28:2085–2088.
Wurst, S. and Van Der Putten, W. H. 2007. Root herbivore identity matters in plant-mediated interactions between root and shoot herbivores. Bas. Appl. Ecol. 8:491–499.
Acknowledgments
We thank E.C. Lampert, A. Trowbridge, J. Paritsis, and S. Whitehead for valuable comments and suggestions on this manuscript. In addition, we acknowledge A. Hill, L. Mulder, E. Burke, and M. Tapy for greenhouse and laboratory assistance; and K.E. Barton for the seeds provided for this study. Funding for this project was provided by the UROP program and the Department of Ecology and Evolutionary Biology at the University of Colorado, and Dissertation Improvement Grant NSF grant DEB 0909717.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quintero, C., Bowers, M.D. Plant Induced Defenses Depend More on Plant Age than Previous History of Damage: Implications for Plant-Herbivore Interactions. J Chem Ecol 37, 992–1001 (2011). https://doi.org/10.1007/s10886-011-0007-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-0007-4