Abstract
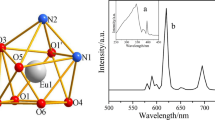
A ligand with two carbonyl groups and one sulfinyl group has been synthesized by a new method and its several lanthanide (III) complexes were synthesized and characterized by element analysis, molar conductivity, coordination titration analysis, IR, TG-DSC, 1H NMR and UV spectra. The results indicated that the composition of these complexes is REL5(ClO4)3·3H2O (RE = La(III), Pr(III), Eu(III), Tb(III), Yb(III), L = C6H5COCH2SOCH2COC6H5). The fluorescent spectra illustrate that both the Tb (III) and Eu (III) complexes display characteristic metal-centered fluorescence in solid state, indicating the ligand favors energy transfer to the excitation state energy level of them. However, the Tb (III) complex displays more effective luminescence than the Eu (III) complex, which is attributed to especial effectively in transferring energy from the average triplet energy level of the ligands (T) onto the excited state (5D4) of Tb (III) than that (5D0) of Eu (III), showing a good antenna effect for Tb(III) luminescence. The phosphorescence spectra and the relationship between fluorescence lifetimes and fluorescence intensities were also discussed.









Similar content being viewed by others
References
Buono-core GE, Li H, Marciniak B (1990) Quenching of excited states by lanthanide ions and chelates in solution. CoordChemRev 90:55–87
Wu W-N, Tang N, Yan L (2008) Syntheses, characterization and fluorescent properties of six novel lanthanide complexes with N,N-diphenyl-2-(quinolin-8-yloxy)acetamide. J Fluoresc 18:101–107
Wang QM, Yan B (2004) Novel luminescent terbium molecularbased hybrids with modified meta-aminobenzoic acid covalently bonded with silica. J Mater Chem 14:2450
Edward A, Chu TY, Claude C, Sokolik I, Okamoto Y, Dorsinville R (1997) Synthesis and characterization of electroluminescent organo-lanthanide(III) complexes. Synthetic metals 84:433–434
Kido J, Nagai K, Okamoto Y (1993) Organic electroluminescent devices using lanthanide complexes. J Alloy Comp 192:30
Yu JB, Zhou L, Zhang HJ, Zheng YX, Li HR, Deng RP, Peng ZP, Li ZF (2005) Efficient electroluminescence from new lanthanide(Eu3 + , Sm3+) complexes. Inorg Chem 44:1611
Kukhta A, Kolesnik E, Grabchev I (2006) Spectral and luminescent properties and electroluminescence of polyvinycarbazole with 1, 8-naphthalimide in the side chain. J Fluoresc 16:375–378
Meares CF, Wensel TG (1984) Metal chelates as probes of biological systems. Acc Chem Res 17:202
Wu F-B, Zhang C (2002) A new europium β-diketon chelate for ultrasensitive time-resolved fluorescence immunoassays. Anal Biochem 311:57–67
Nishioka T, Yuan J, Yamamoto Y, Sumitomo K, Wang Z, Hashino K, Hosoya C, Ikawa K, Wang G, Matsumoto K (2006) New luminescent europium (III) chelates for DNA labeling. Inorg Chem 45:4088–4096
Scott LK, Horrocks WD (1992) Lanthanide ion luminescence as a probe of DNA structure 2. Non-guanine-containing oligomers and nucleotides. J Inorg Biochem 46:193
Niyama E, Brito HF, Cremona M, Teotonio EES, Reyes R, Birto GES, Felinto MCFC (2005) Synthesis and spectroscopic behavior of highly luminescent Eu3+ -dibenzoylmethanate(DBM) complexes with sulfoxide ligands. Spectrochim Acta Part A 61:2643–2649
Lehn J-M (1990) Perspectives in supramolecular chemistry—from molecular recongnition towards molecular information processing and self-organization. Angew Chem Int Ed Engl 29:1304–1319
An B-L, Gong M-L, Li M-X, Zhang J-M, Cheng Z-X (2005) Synthesis and luminescence of a novel conjugated europium complex with 6-aniline carbonyl 2-pyridine carboxylic acid. J Fluoresc 15:613–617
Lv Y, Zhang J, Cao W, Juan Jc, Zhang F, Xu Z (2007) Synthesis and characteristics of a novel rare earth complex of Eu(TTA)2(N-HPA)phen. J photochem photobiol A 188:155–160
Meshkova SB (2000) The dependence of the luminescence intensity of lanthanide complexes with β-diketones on the ligand from. J Fluoresc 10:333–337
Li W-X, Zhang D-F (2002) Synthesis, characterization and fluorescence of phenylcarboxymethyl sulfoxide complexes with lanthanide nitrates. J Rare Earths 20:430–433
Li W-X, Wang H-S, Luo Q-S, Qi Q-G (2004) Synthesis and fluorescence property of Eu3+, Tb3+ perchlorate complexes with diphenyl sulfoxide and 1,10-phenanthroline. J Rare Earths 22:563–566
Shriner RL, Struck HC, Jorison WJ (1930) The preparation and properties of certain sulfoxide and sulfones. Chem Soc 52:2060–2069
Greary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122
Li W-X, Han F-M, Zhao Y-L (2003) Synthesis and characterization of phenyl-phenacyl sulfoxide complexes in heavy rare earth and luminescence of Tb3+ and Eu3+. Chinese Rare Earths 3:11–15
Chen Y, Cai W-M (2005) Synthesis and fluorescence properties of rare earth (Eu3+ and Tb3+) complexes with α-naphthylacetic acid and 1,10-phenanthroline. Spectrochim Acta A 62:863–868
Rosenthal MRJ (1973) The myth of the non-coordinating anion. Chem Educ 50:331–335
Hathaway BJ, Underhill AE (1961) The infrared spectra of some transition-metal perchlorates. J Chem Soc 65:3091–3096
Shen L, Shi M, Shi E-X, Du Y-K, Li F-Y, Huang C-H (2006) Studies on luminescence properties of lanthanide complexes based on pyrazolone. Chem J Chin Univ 27:1413–1417
Qiang S (1993) Chemistry of rare earths. Henan Technology &Science Press, Zhengzhou, pp 304–314 (in Chinese)
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Spectroscopic study of luminescence and intramolecular energy transfer of binary and ternary rare earth complexes with aromatic carboxylic acids and 1,10-phenanthroline. Spectro Lett 31:603
Yan B, Zhang HJ, Wang SB, Ni JZ (1998) Intramolecular Energy transfer mechanism between ligands internary complexes with aromatic acids and 1,10-phenanthroline. J Photochem Photobiol A Chem 116:209
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836
Dean CRS, Shepherd TM (1975) Evaluation of the Intramolecular Energy Transfer Rate Constants in crystalline Eu(hfaa)4ButNH31. J Chem Soc Faraday Trans II 71:146
Gerisler HF, Hellwege KH (1953) Spectrum and luminescent mechanism of crystal Tb(BrO3)3. Physik 136:293–295
Wang Y-G, Gong M-X (1994) Energy transfer from aromatic monoketones to rare earth ions. Acta Scientiarum Naturalium Universitatis Jilinensis 3:89–94
Acknowledgement
The author thanks the financial supports from Inner Mongolia College Scientific Research project (NJ 06047) and Inner Mongolia university ‘513’ second administrative levels person with ability foundation project (205150)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, WX., Guo, L., Chen, LJ. et al. Synthesis and Fluorescence Properties of Lanthanide (III) Perchlorate Complexes with Bis(benzoylmethyl) Sulfoxide. J Fluoresc 18, 1043–1049 (2008). https://doi.org/10.1007/s10895-008-0331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0331-4