Abstract
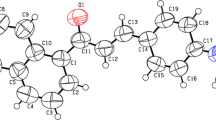
A new chalcone derivative 3-(1-methyl-1H-pyrrol-2-yl)-1-naphthalen-2-yl propenone (MPNP) with electron donor-acceptor group has been synthesized and characterized by IR, 1HNMR, 13C NMR and X- ray crystallography. Electronic absorption and emission spectra of MPNP have been studied in solvents of different polarity. A remarkable red shift was observed in the emission spectrum of MPNP compared to the absorption spectrum upon increasing the solvent polarity, indicating a higher dipole moment in the excited state than in the ground state and the transition involved are π-π* with charge transfer character. Lippert-Mataga and Reichardts correlations were used to estimate the change in dipole moments (Δμ); suggest that the emissive state of MPNP is of strong ICT character. Fluorescence quantum yield (ϕf) of MPNP was correlated with empirical solvent polarity parameter ET(30), and it is observed that ϕf increases with increase in solvent polarity of polar aprotic solvents and decrease in alcoholic solvents. The interaction of MPNP with colloidal silver nanoparticles (AgNPs) was also studied in ethanol and ethylene glycol using steady state emission measurements. The fluorescence quenching data reveal that dynamic quenching and energy transfer play a major role in the fluorescence quenching of MPNP by Ag NPs.












Similar content being viewed by others
References
El-Daly SA, Asiri AM, Alamry KA, Khan SA (2013) Spectroscopic studies and laser activity of 3-(4-dimethylamino-phenyl)-1-(2,5-dimethyl-furan-3-yl)-propenone (DDFP): A new green laser dye. J Lumin 137:6–14
Singh H, Sindhu J, Khurana JM (2014) Determination of dipole moment, solvatochromic studies andapplication as turn off fluorescence chemosensor of new3-(4-(dimethylamino) phenyl)-1-(5-methyl-1-(naphthalen-1-yl)-1H-1,2,3-triazol-4-yl) prop-2-en-1-one. Sensors Actuators B 192:536–542
Rahulan KM, Balamurugan S, Meena KS, Yeap GY, Kanakam CC (2014) Synthesis and nonlinear optical absorption of novel chalcone derivative compounds. Opt Laser Technol 56:142–145
Nagarajan N, Prakash A, Velmurugan G, Shakti N, Katiyar M, Venuvanalingam P, Renganathan R (2014) Synthesis, characterisation and electroluminescence behaviour of π-conjugated imidazole–isoquinoline derivatives. Dyes Pigments 102:180–188
Işık D, Santato C, Barik S, Skene WG (2012) Charge-carrier transport in thin films of π-conjugated thiopheno-azomethines. Org Electron 13(12):3022–3031
Mishra A, Bäuerle P (2012) Small molecule organic semiconductors on the move: promises for future solar energy technology. Angew Chem Int Ed 51(9):2020–2067
SunY CH, Cao D, Liu Z, Chen H, Deng Y, Fang Q (2012) Chalcone derivatives as fluorescence turn-on chemosensors for cyanide anions. J Photochem Photobiol A Chem 244:65–70
Poornesh P, Shettigar S, Umesh G, Manjunatha KB, Prakash Kamath K, Sarojini BK, Narayana B (2009) Nonlinear optical studies on 1, 3-disubstituent chalcones doped polymer films. Opt Mater 31(6):854–859
Rajashekara B, Sowmendranb P, Siva Sankara Sai S, Nageswara Rao G (2012) Synthesis, characterization and two-photon absorption based broadband optical limiting in diarylideneacetone derivative. J Photochem Photobiol A Chem 238:20–23
Chhabra R, Sharma J, Wang H, Zou S, Lin S, Yan H, Liu Y (2009) Distance-dependent interactions between gold nanoparticles and fluorescent molecules with DNA as tunable spacers. Nanotechnology 20(48):485201
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104(1):293–346
Deng W, Goldys EM (2012) Plasmonic approach to enhanced fluorescence for applications in biotechnology and the life sciences. Langmuir 28(27):10152–10163
Hong B, Kang KA (2006) Biocompatible, nanogold-particle fluorescence enhancer for fluorophore mediated, optical immunosensor. Biosens Bioelectron 21(7):1333–1338
Ng MY, Liu WC (2009) Fluorescence enhancements of fiber-optic biosensor with metallic nanoparticles. Opt Express 17(7):5867–5878
Fu Y, Zhang J, Lakowicz JR (2007) Plasmonic enhancement of single-molecule fluorescence near a silver nanoparticle. J Fluoresc 17(6):811–816
Kalele S, Deshpande AC, Singh SB, Kulkarni SK (2008) Tuning luminescence intensity of RHO6G dye using silver nanoparticles. Bull Mater Sci 31(3):541–544
Rainò G, Stöferle T, Park C, Kim HC, Topuria T, Rice PM, Mahrt RF (2011) Plasmonic nanohybrid with ultrasmall Ag nanoparticles and fluorescent dyes. ACS Nano 5(5):3536–3541
Swierczewska M, Lee S, Chen X (2011) The design and application of fluorophore–gold nanoparticle activatable probes. Phys Chem Chem Phys 13(21):9929–9941
Maxwell DJ, Taylor JR, Nie S (2002) Self-assembled nanoparticle probes for recognition and detection of biomolecules. J Am Chem Soc 124(32):9606–9612
Chung HY, Leung PT, Tsai DP (2013) Molecular fluorescence in the vicinity of a charged metallic nanoparticle. Opt Express 21(22):26483–26492
El-Daly SA, Asiri AM, Obeid AY, Khan SA, Alamry KA, Hussien MA, Al-Sehemi AG (2013) Photophysical parameters and laser activity of 3 (4-dimethylamino-phenyl)-1-(2, 5-dimethyl-thiophen-3-yl)-propenone (DDTP): A new potential laser dye. Opt Laser Technol 45:605–612
Sheldrick GM (2007) A short history of SHELX. Acta Crystallogr A: Found Crystallogr 64(1):112–122
Barbour LJ (2001) X-seed— a software tool for supramolecular crystallography. J Supramol Chem 1(4):189–191
Lee PC, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86(17):3391–3395
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3 rd ed. Springer, New York
Jana S, Dalapati S, Ghosh S, Guchhait N (2013) Excited state intramolecular charge transfer process in 5-(4-dimethylamino-phenyl)-penta-2,4-dienoic acid ethyl ester and effect of acceptor functional groups. J Photochem Photobiol A Chem 261:31–40
Shaikh M, Pal H (2014) Photophysics of donor–acceptor kind of styryl dyes: Involvement of twisted intramolecular charge transfer (TICT) state and the effect of solvent polarity. J Spectrosc Dyn 4:1–12
Lippert E (1957) Spectroscopic determination of the dipole moment of aromatic compounds in the first excited singlet state. Z Elektrochem 61:962–975
Mataga N, Kubota T (1970) Molecular interactions and electronic spectra. Marcel Dekker, New York, pp 371–410
Suppan P (1983) Excited-state dipole moments from absorption/fluorescence solvatochromic ratios. Chem Phys Let 94:272–275
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94(8):2319–2358
Ravi M, Soujanya T, Samanta A, Radhakrishnan TP (1995) Excited-state dipole moments of some Coumarin dyes from a solvatochromic method using the solvent polarity parameter, ENT. J Chem Soc Faraday Trans 91(17):2739–2742
Coe BJ, Harris JA, Asselberghs I, Clays K, Olbrechts G, Persoons A, Hupp JT, Johnson RC, Coles SJ, Hursthouse MB, Nakatani K (2002) Quadratic nonlinear optical properties of N-Aryl stilbazolium dyes. Adv Funct Mater 12:110–116
Gordon P, Gregory P (1987) Organic chemistry in colour. Chimia, Moskva
Rurack K, Dekhtyar MI, Bricks JL, Resch-Genger U, Retting W (1999) Quantum yield switching of fluorescence by selectively bridging single and double bonds in chalcones: involvement of two different types of conical intersections. J Phys Chem A 103:9626–1935
Birks JB (1970) Photophysics of aromatic molecules. Wiley Interscience, London, p 88
Förster T (1996). in: O. Sinanoglu (Ed.), Modern Quantum Chemistry, Academic Press, New York.
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Patterns in hydrogen bonding: functionality and graph Set analysis in crystals. Angew Chem Int Ed Engl 34:1555–1573
Acknowledgments
The authors thank the Center of Excellence for Advanced Materials Research and Department of Chemistry at King AbdulAziz University for providing the research facilities. One of the authors, Mehboobali Pannipara is grateful to Deanship of Graduate Studies, King Abdulaziz University for providing PhD Fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Pannipara, M., Asiri, A.M., Alamry, K.A. et al. Spectroscopic Investigation, Effect of Solvent Polarity and Fluorescence Quenching of a New D-π-A Type Chalcone Derivative. J Fluoresc 24, 1629–1638 (2014). https://doi.org/10.1007/s10895-014-1449-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1449-1