Abstract
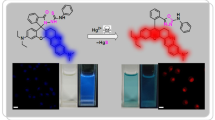
A new turn on fluorescence probe based on 3′,6′-dihydroxy-6-methyl-2-((pyridin-2-ylmethylene)amino)-4-(p-tolyl)spiro[benzo[f]isoindole-1,9′-xanthen]-3(2H)-one (BFFPH) derived from benzo[f]fluorescein was prepared. Full characterization of the prepared probe using spectroscopic analysis was described such as IR, NMR and MS spectra. The sensitivity of BFFPH for monitoring of pH change in alkaline medium was studied. BFFPH exhibited a high sensitivity to alkaline pH by two pKa values at 8.82 and 10.66 in UV/vis spectroscopy titration. The pH monitoring was studied in broad range of pH values (2.5–12.2) at two pKa values at 8.72 and 10.73 by recording the effect of pH on the fluorescence intensity of BFFPH. The acid-base reversibility character of the probe was investigated as well as the effect of the pH change on the fluorescence quantum yield. The application of the prepared BFFPH probe for detection of living Escherichia coli (E. coli) bacteria using confocal fluorescence microscope was investigated.


















Similar content being viewed by others
References
Martínez-Zaguilán R, Chinnock BF, Wald-Hopkins S et al (1996) [Ca2+]i and pHin homeostasis in Kaposi sarcoma cells. Cell Physiol Biochem 6:169–184. https://doi.org/10.1159/000154820
Shimizu Y, Hunt SW (1996) Regulating integrin-mediated adhesion: one more function of PI 3-kinase? Immunol Today 17:565–573. https://doi.org/10.1016/S0167-5699(96)10061-X
Falke JJ, Bass RB, Butler SL, Chervitz SA, Danielson MA (1997) THE TWO-COMPONENT SIGNALING PATHWAY OF BACTERIAL CHEMOTAXIS: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol 13:457–512. https://doi.org/10.1146/annurev.cellbio.13.1.457
Okamoto CT (1998) Endocytosis and transcytosis. Adv Drug Deliv Rev 29:215–228
Satoh H, Hayashi H, Katoh H et al (1995) Na+/H+ and Na+/Ca2+ exchange in regulation of [Na+]i and [Ca2+]i during metabolic inhibition. Am J Physiol Circ Physiol 268:H1239–H1248. https://doi.org/10.1152/ajpheart.1995.268.3.H1239
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent Chemosensors based on Spiroring-opening of Xanthenes and related derivatives. Chem Rev 112:1910–1956. https://doi.org/10.1021/cr200201z
Kogot-Levin A, Zeigler M, Ornoy A, Bach G (2009) Mucolipidosis type IV: the effect of increased Lysosomal pH on the abnormal Lysosomal storage. Pediatr Res 65:686–690
Poschet J, Perkett E, Deretic V (2002) Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mol Med 8:512–519. https://doi.org/10.1016/S1471-4914(02)02414-0
Gameiro P, Reis S, Lima JLFC, de Castro B (2000) Calibration of pH glass electrodes by direct strong acid/strong base titrations under dilute conditions. Anal Chim Acta 405:167–172. Doi. https://doi.org/10.1016/S0003-2670(99)00641-8
Balazs N, Sipos P (2007) Limitations of pH-potentiometric titration for the determination of the degree of deacetylation of chitosan. Carbohydr Res 342:124–130. https://doi.org/10.1016/j.carres.2006.11.016
Kim TH, Kim SH, Van Tan L et al (2008) Diazo-coupled calix[4]arenes for qualitative analytical screening of metal ions. Talanta 74:1654–1658. https://doi.org/10.1016/j.talanta.2007.10.033
Qian YA, Cao L, Jia CM et al (2015) A highly selective chemosensor for naked-eye sensing of nanomolar cu(II) in an aqueous medium. RSC Adv 5:77965–77972. https://doi.org/10.1039/c5ra12407g
Chen XQ, Pradhan T, Wang F et al (2012) Fluorescent Chemosensors based on Spiroring-opening of Xanthenes and related derivatives. Chem Rev 112:1910–1956. https://doi.org/10.1021/cr200201z
Aysha TS, El-Sedik MS, Mohamed MBI, et al (2019) Dual functional colorimetric and turn-off fluorescence probe based on pyrrolinone ester hydrazone dye derivative for cu 2+ monitoring and pH change. Dye pigment 170:. 10.1016/j.dyepig.2019.107549
Elmorsi TM, Aysha TS, Machalický O et al (2017) A dual functional colorimetric and fluorescence chemosensor based on benzo[f]fluorescein dye derivatives for copper ions and pH; kinetics and thermodynamic study. Sensors Actuators B Chem 253:437–450. https://doi.org/10.1016/j.snb.2017.06.084
Elmorsi TM, Aysha TS, Sheier MB, Bedair AH (2017) Synthesis, kinetics, and equilibrium study of highly sensitive colorimetric Chemosensor for monitoring of copper ions based on Benzo[f]fluorescein dye derivatives. Zeitschrift fur Anorg und Allg Chemie 643:811–818
Eldessouki M, Aysha T, Ratičáková M et al (2017) Structural parameters of functional membranes for integration in smart wearable materials. Fibres Text East Eur 25:73–78. https://doi.org/10.5604/01.3001.0010.4631
Shi W, He S, Wei M et al (2010) Optical pH sensor with rapid response based on a fluorescein-intercalated layered double hydroxide. Adv Funct Mater 20:3856–3863. https://doi.org/10.1002/adfm.201001081
Andersson RM, Carlsson K, Liljeborg A, Brismar H (2000) Characterization of probe binding and comparison of its influence on fluorescence lifetime of two pH-sensitive benzo [c] xanthene dyes using intensity-modulated multiple-wavelength scanning technique. Anal Biochem 283:104–110
Xie J, Chen Y, Yang W et al (2011) Water soluble 1,8-naphthalimide fluorescent pH probes and their application to bioimagings. J Photochem Photobiol A Chem 223:111–118. https://doi.org/10.1016/j.jphotochem.2011.08.006
Baruah M, Qin W, Basarić N, de Borggraeve WM, Boens N (2005) BODIPY-based Hydroxyaryl derivatives as fluorescent pH probes. J Org Chem 70:4152–4157. https://doi.org/10.1021/jo0503714
Wang KP, Jin ZH, Shang HS, Lv CD, Zhang Q, Chen S, Hu ZQ (2017) A highly selective fluorescent Chemosensor for Zn2+ based on the Rhodamine derivative incorporating Coumarin group. J Fluoresc 27:629–633. https://doi.org/10.1007/s10895-016-1991-0
Chiou YR, Wan CF, Wu AT (2017) A selective colorimetric and turn-on fluorescent Chemosensor for Hg2+ in aqueous solution. J Fluoresc 27:317–322. https://doi.org/10.1007/s10895-016-1960-7
Quy PT, Hien NK, Bao NC, Nhan DT, Khanh DV, Nhung NT, Tung TQ, Luyen ND, Quang DT (2015) A new rhodamine-based fluorescent chemodosimeter for mercuric ions in water media. Luminescence 30:325–329. https://doi.org/10.1002/bio.2733
Czaplyski WL, Purnell GE, Roberts CA, Allred RM, Harbron EJ (2014) Substituent effects on the turn-on kinetics of rhodamine-based fluorescent pH probes. Org Biomol Chem 12:526–533. https://doi.org/10.1039/c3ob42089b
Yang Y, Yin C, Huo F et al (2013) pH-sensitive fluorescent salicylaldehyde derivative for selective imaging of hydrogen sulfide in living cells. Sensors Actuators B Chem 186:212–218. https://doi.org/10.1016/j.snb.2013.06.013
Chang MCY, Pralle A, Isacoff EY, Chang CJ (2004) A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 126:15392–15393. https://doi.org/10.1021/ja0441716
Yu FB, Zhang WS, Li P et al (2009) Cu(2+)-selective naked-eye and fluorescent probe: its crystal structure and application in bioimaging. Analyst 134:1826–1833. https://doi.org/10.1039/b823360h
Zhang B, Diao Q, Ma P et al (2016) A sensitive fluorescent probe for Cu2+ based on rhodamine B derivatives and its application to drinking water examination and living cells imaging. Sensors Actuators B Chem 225:579–585. https://doi.org/10.1016/j.snb.2015.11.069
Lin WY, Long LL, Chen BB, Tan W, Gao W (2010) Fluorescence turn-on detection of Cu2+ in water samples and living cells based on the unprecedented copper-mediated dihydrorosamine oxidation reaction. Chem Commun 46:1311–1313. https://doi.org/10.1039/b919531a
Tang X, Zhu Z, Wang Y et al (2018) A dual site controlled probe for fluorescent monitoring of intracellular pH and colorimetric monitoring of Cu2+. Sensors Actuators B Chem 270:35–44. https://doi.org/10.1016/j.snb.2018.04.173
Omara ST (2017) MIC and MBC of honey and gold nanoparticles against methicillin-resistant (MRSA) and vancomycin-resistant (VRSA) coagulase-positive S. aureus isolated from contagious bovine clinical mastitis. J Genet Eng Biotechnol 15:219–230. https://doi.org/10.1016/J.JGEB.2017.02.010
Wall SK, Hernández-Castellano LE, Ahmadpour A, Bruckmaier RM, Wellnitz O (2016) Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. J Dairy Sci 99:7544–7553. https://doi.org/10.3168/JDS.2016-11093
Kusebauch U, Hernández-Castellano LE, Bislev SL, Moritz RL, Røntved CM, Bendixen E (2018) Selected reaction monitoring mass spectrometry of mastitis milk reveals pathogen-specific regulation of bovine host response proteins. J Dairy Sci 101:6532–6541. https://doi.org/10.3168/JDS.2017-14312
Gonçalves JL, Kamphuis C, Martins CMMR et al (2018) Bovine subclinical mastitis reduces milk yield and economic return. Livest Sci 210:25–32. https://doi.org/10.1016/j.livsci.2018.01.016
Blood DC, Radostits OM, Gay (2017) Veterinary medicine a textbook of the diseases of cattle, Sheep, Pigs, Goats and Horses
Moreira LH, de Souza JCP, de Lima CJ, Salgado MAC, Fernandes AB, Andreani DIK, Villaverde AB, Zângaro RA (2018) Use of photodynamic therapy in the treatment of bovine subclinical mastitis. Photodiagn Photodyn Ther 21:246–251. https://doi.org/10.1016/J.PDPDT.2017.12.009
Baddar FG, El-Assal LS, Doss NA (1955) Phenylpropiolic acids. Part IV The self-condensation of o- and p-tolyl- and o-chlorophenyl-propiolic acids. J Chem Soc 0:461–465. https://doi.org/10.1039/JR9550000461
Britton HTS, Robinson RA (1931) CXCVIII.—universal buffer solutions and the dissociation constant of veronal. J Chem Soc 0:1456–1462. https://doi.org/10.1039/JR9310001456
Liu L, Guo P, Chai L et al (2014) Fluorescent and colorimetric detection of pH by a rhodamine-based probe. Sensors Actuators B Chem 194:498–502. https://doi.org/10.1016/j.snb.2013.12.023
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Springer US
Chao J, Song K, Zhang Y, Yin C, Huo F, Wang J, Zhang T (2018) A pyrene-based colorimetric and fluorescent pH probe with large stokes shift and its application in bioimaging. Talanta 189:150–156. https://doi.org/10.1016/J.TALANTA.2018.06.073
Artursson K, Schelin J, Lambertz ST et al (2018) Foodborne pathogens in unpasteurized milk in Sweden. Int J Food Microbiol 284:120–127. https://doi.org/10.1016/j.ijfoodmicro.2018.05.015
Dohoo IR, Smith J, Andersen S, Kelton DF, Godden S, Mastitis Research Workers' Conference (2011) Diagnosing intramammary infections: evaluation of definitions based on a single milk sample. J Dairy Sci 94:250–261. https://doi.org/10.3168/JDS.2010-3559
Griffio K, Velthuis AGJ, Lagerwerf LA et al (2018) Agreement between four commercial diagnostic tests and routine bacteriological culture of milk to determine the udder infection status of dairy cows. Prev Vet Med J 157:162–173. https://doi.org/10.1016/j.prevetmed.2018.07.003
Acknowledgements
We gratefully thanks National Research Centre, Giza, Egypt through project 11070103 (colorimetric chemosensors and fluorescence probes: a promising strategy for environmental monitoring of ionic species and toxic gases) for the financial support. We could not forget to thank the centre of excellence for advances sciences, National Research Centre and the central laboratory of textile research division for allowing us to use all their apparatus. We also thank the Sensors-Probes and Devices Lab, Chemistry Department, Faculty of Science, Al-Azhar University to produce this work. Also we thank Prof. Dr. Omaima M. Kandil director to Embryo and Genetic Resource Conservation Bank in National Research Centre, financially supported by STDF (C.B. grant ID: 2339) for her work in preparation of samples for image application using confocal microscope zeiss LSM 710 photos.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamed, M.B.I., Aysha, T.S., Elmorsi, T.M. et al. Colorimetric Chemosensor and Turn on Fluorescence Probe for pH Monitoring Based on Xanthene Dye Derivatives and its Bioimaging of Living Escherichia coli Bacteria. J Fluoresc 30, 601–612 (2020). https://doi.org/10.1007/s10895-020-02522-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02522-1