Abstract
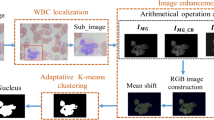
Blood leucocytes segmentation in medical images is viewed as difficult process due to the variability of blood cells concerning their shape and size and the difficulty towards determining location of Blood Leucocytes. Physical analysis of blood tests to recognize leukocytes is tedious, time-consuming and liable to error because of the various morphological components of the cells. Segmentation of medical imagery has been considered as a difficult task because of complexity of images, and also due to the non-availability of leucocytes models which entirely captures the probable shapes in each structures and also incorporate cell overlapping, the expansive variety of the blood cells concerning their shape and size, various elements influencing the outer appearance of the blood leucocytes, and low Static Microscope Image disparity from extra issues outcoming about because of noise. We suggest a strategy towards segmentation of blood leucocytes using static microscope images which is a resultant of three prevailing systems of computer vision fiction: enhancing the image, Support vector machine for segmenting the image, and filtering out non ROI (region of interest) on the basis of Local binary patterns and texture features. Every one of these strategies are modified for blood leucocytes division issue, in this manner the subsequent techniques are very vigorous when compared with its individual segments. Eventually, we assess framework based by compare the outcome and manual division. The findings outcome from this study have shown a new approach that automatically segments the blood leucocytes and identify it from a static microscope images. Initially, the method uses a trainable segmentation procedure and trained support vector machine classifier to accurately identify the position of the ROI. After that, filtering out non ROI have proposed based on histogram analysis to avoid the non ROI and chose the right object. Finally, identify the blood leucocytes type using the texture feature. The performance of the foreseen approach has been tried in appearing differently in relation to the system against manual examination by a gynaecologist utilizing diverse scales. A total of 100 microscope images were used for the comparison, and the results showed that the proposed solution is a viable alternative to the manual segmentation method for accurately determining the ROI. We have evaluated the blood leucocytes identification using the ROI texture (LBP Feature). The identification accuracy in the technique used is about 95.3%., with 100 sensitivity and 91.66% specificity.







Similar content being viewed by others
References
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., and Ibrahim, D. A., Review on Nasopharyngeal Carcinoma: Concepts, methods of analysis, segmentation, classification, prediction and impact: A review of the research literature. J. Comput. Sci. 21:283–298, 2017.
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., and Ibrahim, D. A., Analysis of an electronic methods for nasopharyngeal carcinoma: Prevalence, diagnosis, challenges and technologies. J. Comput. Sci. 21:241–254, 2017.
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., Abdullah, M. K., and Ibrahim, D. A., Automatic segmentation and automatic seed point selection of nasopharyngeal carcinoma from microscopy images using region growing based approach. J. Comput. Sci. 20:61–69, 2017.
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., Ibrahim, D. A., and Abdullah, M. K., Artificial neural networks for automatic segmentation and identification of nasopharyngeal carcinoma. J. Comput. Sci. 21:263–274, 2017.
Saraswat, M., and Arya, K. V., Automated microscopic image analysis for leukocytes identification: A survey. Micron 65:20–33, 2014.
Khashman, A., Blood cell identification using emotional neural networks. J. Inf. Sci. Eng. 25(6):1737–1751, 2009.
Habibzadeh, M., Krzyżak, A. and Fevens, T., Comparative study of feature selection for white blood cell differential counts in low resolution images. In IAPR Workshop on Artificial Neural Networks in Pattern Recognition (pp. 216–227). Springer, Cham, 2014.
Savkare, S. S., and Narote, S. P., Automatic classification of normal and infected blood cells for parasitemia detection. Int J Comput Sci Net Sec 11:94–97, 2011.
Adollah, R., Mashor, M.Y., Nasir, N.M., Rosline, H., Mahsin, H., and Adilah, H., Blood cell image segmentation: a review. In 4th Kuala Lumpur International Conference on Biomedical Engineering 2008 (pp. 141–144). Springer, Berlin, Heidelberg, 2008.
Ingram, M., and Preston, K., Automatic analysis of blood cells. Sci. Am. 223(5):72–83, 1970.
Young, I. T., The classification of white blood cells. IEEE Trans. Biomed. Eng. 4:291–298, 1972.
Ongun, G., Halici, U., Leblebicioğlu, K., Atalay, V., Beksaç, S., and Beksaç, M., Automated contour detection in blood cell images by an efficient snake algorithm. Nonlinear Anal. Theory Methods Appl. 47(9):5839–5847, 2001.
Wang, X., He, L., and Wee, W., Deformable contour method: a constrained optimization approach. Int. J. Comput. Vis. 59(1):87–108, 2004.
Bikhet, S.F., Darwish, A.M., Tolba, H.A. and Shaheen, S.I., Segmentation and classification of white blood cells. In Acoustics, Speech, and Signal Processing, 2000. ICASSP'00. Proceedings. 2000 I.E. International Conference on (Vol. 4, pp. 2259–2261). IEEE, 2000.
Theera-Umpon, N., Patch-based white blood cell nucleus segmentation using fuzzy clustering. ECTI-EEC 3(1):15–19, 2005.
Jiang, K., Liao, Q.M. and Dai, S.Y., A novel white blood cell segmentation scheme using scale-space filtering and watershed clustering. In Machine Learning and Cybernetics, 2003 International Conference on (Vol. 5, pp. 2820–2825). IEEE, 2003.
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., Mostafa, S. A., Ibrahim, D. A., Jameel, H. K., and Alallah, A. H., Solving vehicle routing problem by using improved K-nearest neighbor algorithm for best solution. J. Comput. Sci. 21:232–240, 2017.
Mohammed, M. A., Ghani, M. K. A., Hamed, R. I., Mostafa, S. A., Ahmad, M. S., and Ibrahim, D. A., Solving vehicle routing problem by using improved genetic algorithm for optimal solution. J. Comput. Sci. 21:255–262, 2017.
Piuri, V., and Scotti, F., Morphological classification of blood leucocytes by microscope images. In Computational Intelligence for Measurement Systems and Applications, 2004. CIMSA. 2004 I.E. International Conference on (pp. 103–108). IEEE, 2004.
Scotti, F., Automatic morphological analysis for acute leukemia identification in peripheral blood microscope images. In Computational Intelligence for Measurement Systems and Applications, 2005. CIMSA. 2005 I.E. International Conference on (pp. 96–101). IEEE, 2005.
Labati, R.D., Piuri, V., and Scotti, F., All-IDB: The acute lymphoblastic leukemia image database for image processing. In Image processing (ICIP), 2011 18th IEEE international conference on (pp. 2045–2048). IEEE, 2011.
Scotti, F., Robust segmentation and measurements techniques of white cells in blood microscope images. In Instrumentation and Measurement Technology Conference, 2006. IMTC 2006. Proceedings of the IEEE (pp. 43–48). IEEE, 2006.
Koltsov, P. P., Kotovich, N. V., Kravchenko, A. A., Kutsaev, A. S., Kuznetsov, A. B., Osipov, A. S., Sukhenko, E. P., and Zakharov, A. V., A segmentation method for the microscopy of images of blood cells. Pattern Recognit. Image Anal. 25(2):167–173, 2015.
Mohammed, M.A., Al-Khateeb, B., and Ibrahim, D.A., Case based reasoning shell frameworkas decision support tool. Indian Journal of Science and Technology, 9(42), 2016.
Mohammed, M.A., Ahmad, M.S., and Mostafa, S.A., Using genetic algorithm in implementing capacitated vehicle routing problem. In Computer & Information Science (ICCIS), 2012 International Conference on (Vol. 1, pp. 257–262). IEEE, 2012.
Ng, P. E., and Ma, K. K., A switching median filter with boundary discriminative noise detection for extremely corrupted images. IEEE Trans. Image Process. 15(6):1506–1516, 2006.
Loupas, T., McDicken, W. N., and Allan, P. L., An adaptive weighted median filter for speckle suppression in medical ultrasonic images. IEEE Trans. Circuits Syst. 36(1):129–135, 1989.
Ghani, M.K.A., Mohammed, M.A., Ibrahim, M.S., Mostafa, S.A., and Ibrahim, D.A., Implementing an efficient expert system for services center management by fuzzy logic controller. Journal of Theoretical & Applied Information Technology, 95 (13), 2017.
Mäenpää, T., 2003. The local binary pattern approach to texture analysis: extensions and applications (pp. 42–47). Oulun yliopisto.
Wang, Y., Wei, X., and Xiao, S., LBP texture analysis based on the local adaptive Niblack algorithm. In Image and Signal Processing, 2008. CISP'08. Congress on (Vol. 2, pp. 777–780). IEEE, 2008.
Chan, H. P., Sahiner, B., Lam, K. L., Petrick, N., Helvie, M. A., Goodsitt, M. M., and Adler, D. D., Computerized analysis of mammographic microcalcifications in morphological and texture feature spaces. Med. Phys. 25(10):2007–2019, 1998.
Sahiner, B., Chan, H. P., Petrick, N., Helvie, M. A., and Hadjiiski, L. M., Improvement of mammographic mass characterization using spiculation measures and morphological features. Med. Phys. 28(7):1455–1465, 2001.
Yang, A. Y., Wright, J., Ma, Y., and Sastry, S. S., Unsupervised segmentation of natural images via lossy data compression. Comput. Vis. Image Underst. 110(2):212–225, 2008.
Binder, T., Süssner, M., Moertl, D., Strohmer, T., Baumgartner, H., Maurer, G., and Porenta, G., Artificial neural networks and spatial temporal contour linking for automated endocardial contour detection on echocardiograms: A novel approach to determine left ventricular contractile function. Ultrasound Med. Biol. 25(7):1069–1076, 1999.
Wang, Z., Bovik, A. C., Sheikh, H. R., and Simoncelli, E. P., Image quality assessment: from error visibility to structural similarity. IEEE Trans. Image Process. 13(4):600–612, 2004.
Kaur, J., Agrawal, S., and Vig, R., Integration of clustering, optimization and partial differential equation method for improved image segmentation. Int. J. Image, Graph. Signal Process. 4(11):26–33, 2012.
Meil, M., Comparing Clusterings – An Information Based Distance. 98:873–895, 2007.
Martin, D., Fowlkes,C., Tal, D., Malik, J., A database of human segmented natural images and its application to evaluating segmentation algorithms and measuring ecological statistics, Proc. Eighth IEEE Int. Conf. Comput. Vision. ICCV 2, 2001.
Zhang, R., Shen, J., Wei, F., Li, X., and Sangaiah, A. K., Medical image classification based on multi-scale non-negative sparse coding. Artif. Intell. Med., 2017. https://doi.org/10.1016/j.artmed.2017.05.006.
Liao, X., Yin, J., Guo, S., Li, X., and Sangaiah, A. K., Medical JPEG image steganography based on preserving inter-block dependencies. Comput. Electr. Eng., 2017. https://doi.org/10.1016/j.compeleceng.2017.08.020.
Zheng, H. T., Wang, Z., Ma, N., Chen, J., Xiao, X., and Sangaiah, A. K., Weakly-supervised image captioning based on rich contextual information. Multimedia Tools and Applications:1–17, 2017. https://doi.org/10.1007/s11042-017-5236-2.
Vinh, N. X., Epps, J., and Bailey, J., Information theoretic measures for clusterings comparison: Variants, properties, normalization and correction for chance. J. Mach. Learn. Res. 11:2837–2854, 2010.
Zhou, D., Bousquet, O., Lal, T.N., Weston, J. and Schölkopf, B., Learning with local and global consistency. In Advances in neural information processing systems (pp. 321–328), 2004.
Sangaiah, A. K., Samuel, O. W., Li, X., Abdel-Basset, M., and Wang, H., Towards an efficient risk assessment in software projects–Fuzzy reinforcement paradigm. Computers & Electrical Engineering, Elsevier Publishers., 2017. https://doi.org/10.1016/j.compeleceng.2017.07.022.
Sangaiah, A. K., Thangavelu, A. K., Gao, X. Z., Anbazhagan, N., and Durai, M. S., An ANFIS approach for evaluation of team-level service climate in GSD projects using Taguchi-genetic learning algorithm. Applied Soft Computing, Elsevier Publishers 30:628–635, 2015.
Medhane, D. V., and Sangaiah, A. K., ESCAPE: Effective Scalable Clustering Approach for Parallel Execution of continuous position-based queries in position monitoring applications. IEEE Transactions on Sustainable Computing., 2017. https://doi.org/10.1109/TSUSC.2017.2690378.
Abdel-Basset, M., Fakhry, A. E., El-henawy, I., Qiu, T., and Sangaiah, A. K., Feature and Intensity Based Medical Image Registration Using Particle Swarm Optimization. J. Med. Syst. 41(12):197, 2017. https://doi.org/10.1007/s10916-017-0846-9.
Yao, S., Sangaiah, A. K., Zheng, Z., and Wang, T., Sparsity estimation matching pursuit algorithm based on restricted isometry property for signal reconstruction. Future Generation Computer Systems. Elsevier Publishers. https://doi.org/10.1016/j.future.2017.09.034, 2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Image & Signal Processing
Rights and permissions
About this article
Cite this article
Abdulhay, E., Mohammed, M.A., Ibrahim, D.A. et al. Computer Aided Solution for Automatic Segmenting and Measurements of Blood Leucocytes Using Static Microscope Images . J Med Syst 42, 58 (2018). https://doi.org/10.1007/s10916-018-0912-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-018-0912-y