Abstract
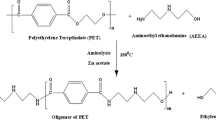
Chemical recycling of PET has been developed by various methods. Aminolysis is one of chemical recycling methods of PET has been developed recently. The obtained product using aminolysis, Bis (2-hydroxy ethylene) terephthalamide (BHETA), has the potential for further reactions to obtain useful products. There are few reports on usage of recycled BHETA from PET waste to synthesis of polyurethanes. On the other hand, various biodegradable polyurethanes have been synthesized using polycaprolactone diol. Therefore, caprolactone is a new potential in synthesis of biodegradable polyurethanes from PET waste. In this work, novel biodegradable polyurethanes have been synthesized using BHETA. In this order, at first polyols with different molecular weights have been synthesized through ring opening polymerization of caprolactone by BHETA, then urethane linkages were formed using HDI (Hexamethylene Diisocyanate) without chain extender. Chemical, thermal, mechanical and dynamic mechanical properties, biodegradability, morphology and UV resistance of synthesized polyurethanes have been investigated.














Similar content being viewed by others
References
Kloss J, Fernanda SM, Souza D, Edilsa R, Silva D, Jair Alves D, Leni A, Soˆnia Faria Z (2006) Macromol Symp 245–246: 651–656
Shukla SR, Harad AM, Jawale LS (2009) Polym Deg Stab 94:604–609
Sivaram S (1997) National seminar on recycling and plastics waste management 24–26 Sep, pp 283–288
Barbozaa ES, Lopez DR, Amico SC, Ferreira CA (2009) Resour Concerv Recy 53:122
Pusztaszeri SF (1982) US Patent 4355175
Mishra S, Goje AS, Zope VS (2003) Poly Plastics Technol Eng 42(4):581–603
Mishra S, Goje AS, Zope VS (2003) Polym React Eng 11(1):79–99
Schwartz J (1995) US Patent 5395858
Lamparter RA, Barna BA, Johnsrud DR (1985) US Patent 4542239
Tindall GW, Perry RL (1991) US Patent 5045122
Mishra S, Goje AS (2003) Polym React Eng 11(4):963–987
Doerr ML (1986) US Patent 4578510
Yang Y, Lu Y, Xiang H, Xu Y, Li Y (2002) Polym Degrad Stabil 75:185–191
Motonobu G, Hiroshi K, Akio K, Tsutomu H, Shoji N (2002) J Phys Conden Matter 14(44):11427–11430
Motonobu G, Hiroshi K, Akio K, Tsutomu H, Shoji N, McCoy BJ (2002) Alche J 48(1):136–144
Akiharu F, Minako S, Masashige M (1986) US Patent 4609680
Ostrowski HS (1975) US Patent 3884850
Güçlü G, Kasgöz A, Özbudak S, Özgümüs S, Orbay M (1998) J Appl Polym Sci 69(12):23–2319
Andrej K (1998) J Appl Polym Sci 69(6–8):1115–1118
Berti C, Colonna M, Fiorini M, Lorenzetti C, Marchese P (2004) Macromol Mater Eng 289:49–55
Manfred K, Wolfgang S, Uwe S (1993) US Patent 5266601
Shukla SR, Harad AM (2006) Polym Degrad Stab 91:1850–1854
Fabrycy E, Leistner A, Spychaj T (2000) Adhesion 44(4):35
Zahn H, Pfeifer H (1963) Polymer 4:429–432
Popoola V (1998) J Appl Polym Sci 36:1677–1683
Blackmon KP, Fox DW, Shafer SJ (1990) US Patent 4973746
Shamsi R, Abdouss M, Mir Mohamad Sadeghi G, Afshar Taromi F (2009) Polym Int 58:22–30
Aslzadeh MM, Mir M Sadeghi G, Abdouss M (2010) Mat-Wiss U Werkstofftech 41(8): 682–688
Yeganeh H, Jamshidi H, Jamshidi S (2007) Polym Int 56:41–49
Heijkantsa R, Calcka R, Tienenb T, Groota J, Bumab P, Penningsa A, Veth RPH (2005) AJ Schouten: Biomat 26:4219–4228
Borda J, Ke′ki S, Bodna′r I, Ne′meth N, Zsuga M (2006) Polym Adv Technol 17: 945–953
Liu C, Gu Y, Qian Z, Fan L, Li J, Chao G, Tu M, Jia W (2005) J Biomed Mat Res 75A:465–471
Cohn D, Stern T, Gonza′lez M, Epstein JJ (2002) Biomed Mat Res 59:273–281
Jiang X, Li J, Ding M, Tan H, Ling Q, Zhong Y, Fu Q (2007) Eur Polym J 43:1838–1846
Xie Z, Lu C, Chen X, Chen L, Hu X, Shi Q, Jing X (2007) Eur Polym J 43:2080–2087
Gorna K, Gogolewski S (2002) Polym Degrad Stabil 75:113–122
Yen M, Kuo S (1998) J Polym Res 5:125–131
Wang W, Ping P, Yu H, Chen X, Jing X (2006) J Polym Sci Pol Chem 44:5505–5512
Nagata M, Kato K, Sakai W, Tsutsumi N (2006) Macromol Biosci 6:333–339
Kloss J, Souza F, Silva E, Dionı′sio J, Akcelrud L, Zawadzki S (2006) Macromol Symp 245–246:651–656
Hassan MK, Mauritz KA, Storey RF, Wiggins JS (2006) J Polym Sci Pol Chem 44:2990–3000
Gorna K, Polowinski S, Gogolewski S (2002) J Polym Sci Pol Chem 40:156–170
Skarja GA, Woodhouse KA (2000) J Appl Polym Sci 75:1522–1534
Ping P, Wang W, Chen X, Jing X (2007) J Polym Sci Pol Phys 45:557–570
Lusinchi JM, Pietrasanta Y, Robin JJ, Boutevin B (1998) J Appl Polym Sci 69:657–665
Magnusson AB (1967) J Appl Polym Sci 11:2175–2188
Zia KM, Barikani M, Bhatti IA, Zuber M, Bhatti HN (2008) J Appl Polym Sci 110:769–776
Szycher S (1999) Handbook of polyurethanes, Chap. 11. CRC Press, FL
Cooper W, Pearson RW, Darke S (1960) The Industrial chemist. pp 121–126
Gogolewski S (1989) Colloid Polym Sci 267:757–785
Petrovic ZS, Ferguson J (1991) J Prog Polym Sci 16:695–836
Mark HF (1998) Ency Polym Sci Tech, 2nd ed. Wiley, New York, 1988:13
Kogon IC (1961) J Org Chem 26:3004–3005
Ulrich HJ (1976) J Polym Sci Macroml Rev 11:93–133
Lyman DJ (1966) Rev Macromol Chem 1: 191–196
Data Sheet of polycaprolactone product, Sigma-Aldrich, www.sigma-aldrich.com
Wunderlich B (1980) Macromolecular physics, crystal melting. Academic Press, New York
Kloss J, Munaro M, De Souza GP, Gulmine JV, Wang SH, Zawadzki S, Akcelrud L (2002) J Polym Sci Part A Polym Chem 40:4117–4130
Downing JW, Newell JA (2004) J Appl Polym Sci 91:417–424
Rein G (2005) Ph.D. Thesis, Uni Cal. Berkeley, http://repositories.cdlib.org/cpl/fs/ReinPhD05
Semsarzadeh MA, Navarchian AH (2003) J Appl Polym Sci 90:963–972
Van Bogart JWC, Gibson PE, Cooper SL (1983) J Polym Sci Polym Phy 21:65–95
Van Bogart JWC, Rluemke DA, Cooper SL (1981) Polymer 22:1428
Von Jacobs H, Jenckel E (1961) Makromol Chem 43:132
Seefried CG, Koleske JV, Critchfield FE (1975) J App Polym Sci 19:2493–2502
Izuka A, Winter HH, Hashimoto T (1992) Macromolecules 25:2422–2428
Heijkants RGJC, Schwab LW, Van Calck RV, De Groot JH, Pennings AJ, Schouten AJ (2005) Polymer 4:8981–8989
Rutkowska M, Krasowska K, Heimowska A, Steinka L, Janik H, Haponiuk J, Karlsson S (2002) Pol J Envir Stu 11(4):413–420
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mir Mohamad Sadeghi, G., Shamsi, R. & Sayaf, M. From Aminolysis Product of PET Waste to Novel Biodegradable Polyurethanes. J Polym Environ 19, 522–534 (2011). https://doi.org/10.1007/s10924-011-0283-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-011-0283-7