Abstract
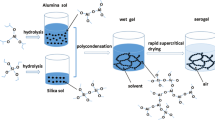
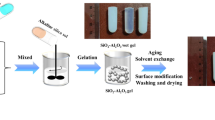
A facile, solvent-deficient, one-pot synthesis of a thermally stable silica-doped alumina, having high surface area, large pore volume and uniquely large pores, has been developed. Silica-doped alumina (SDA) was synthesized by adding 5 wt% silica from tetraethyl orthosilicate (TEOS) to aluminum isoproxide (AIP), a 1:5 mol ratio AIP to water, and a 1:2 mol ratio TEOS to water in the absence of a template. The structure of silica-doped alumina was studied by in situ high-temperature powder XRD, nitrogen adsorption, thermogravimetric analysis, solid-state NMR, and TEM. The addition of silica significantly increases the stability of γ-Al2O3 phase to 1200 °C while maintaining a high surface area, a large pore volume and a large pore diameter. After calcination at 1100 °C for 2 h, a surface area of 160 m2/g, pore volume of 0.99 cm3/g, and a bimodal pore size distribution of 23 and 52 nm are observed. Compared to a commercial silica-doped alumina, after calcination for 24 h at 1100 °C, the surface area, pore volume, and pore diameter SDA are higher by 46, 155, and 94 %, respectively. Results reveal that Si stabilizes the porous structure of γ-Al2O3 up to 1200 °C, while unstabilized alumina is stable to only 900 °C. From our data, we infer that Si enters tetrahedral vacancies in the defect spinel structure of alumina without moving Al from tetrahedral positions and forms a silica–alumina interface.








Similar content being viewed by others
References
M. Ozawa, Thermal stabilization of catalytic compositions for automobile exhaust treatment through rare earth modification of alumina nanoparticle support. J. Alloys Compd. 408–412, 1090–1095 (2006)
H. Pines, W.O. Haag, Alumina: catalyst and support. IX. The alumina catalyzed dehydration of alcohols. J. Am. Chem. Soc. 83, 2847–2852 (1961)
O.V. Klimov, M.A. Fedotov, A.V. Pashigreva, S.V. Budukva, E.N. Kirichenko, G.A. Bukhtiyarova, A.S. Noskov, Complexes forming from ammonium paramolybdate, orthophosphoric acid, cobalt or nickel nitrate, and carbamide in solution and their use in the preparation of diesel fuel hydrodesulfurization catalysts. Kinet. Catal. 50, 867–873 (2009)
C.H. Bartholomew, R.J. Farrauto, Fundamentals of Industrial Catalytic Processes, 2nd edn. (John Wiley & Sons Inc., Hoboken, NJ, 2006)
A.R. de la Osa, A. de Lucas, A. Romero, J.L. Valverde, P. Sanchez, Influence of the catalytic support on the industrial Fischer–Tropsch synthetic diesel production. Catal. Today 176, 298–302 (2011)
B.F. Woodfield, S. Liu, J. Boerio-Goates, Q. Liu, Preparation of Uniform Nanoparticles of Ultra-High Purity Metal Oxides, Mixed Metal Oxides, Metals, and Metal Alloys II, 2006
B.F. Woodfield, C.H. Bartholomew, B. Huang, R.E. Olsen, L. Astle, Method for making highly porous, stable metal oxide with a controlled pore structure, vol. 40, (WO Application Brigham Young University, 2011)
B. Huang, C.H. Bartholonew, S.J. Smith, B.F. Woodfield, Facile solvent-deficient synthesis of mesoporous gamma alumina with controlled pore structures. Microporous Mesoporous Mater. 165, 70–78 (2013)
J. Cejka, Organized mesoporous alumina: synthesis, structure and potential in catalysis. Appl. Catal. A 254, 327–338 (2003)
C. Morterra, G. Magnacca, A case study: surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 27, 497–532 (1996)
R.K. Oberlander, Aluminas for catalysts: their preparation and properties. Appl. Ind. Catal. 3, 63–112 (1984)
J.W. Curley, M.J. Dreelan, O.E. Finlayson, High temperature stability of alumina fiber. Catal. Today 10, 401–404 (1991)
T. Fukui, M. Hori, Thermal stability of aluminas by hydrothermal treatment of an alkoxide-derived gel. J. Mater. Sci. 30, 1794–1800 (1995)
T. Horiuchi, T. Osaki, T. Sugiyama, H. Masuda, M. Horio, K. Suzuki, T. Mori, T. Sago, High surface area alumina aerogel at elevated temperatures. J. Chem. Soc. Faraday Trans. 90, 2573–2578 (1994)
J.B. Miller, E.I. Ko, A homogeneously dispersed silica dopant for control of the textural and structural evolution of an alumina aerogel. Catal. Today 43, 51–67 (1998)
A.A. Shutilov, G.A. Zenkovets, S.V. Tsybulya, V.Y. Gavrilov, Effect of silica on the stability of the nanostructure and texture of fine-particle alumina. Kinet. Catal. 53, 125–136 (2012)
T. Horiuchi, L. Chen, T. Osaki, T. Sugiyama, K. Suzuki, T. Mori, A novel alumina catalyst support with high thermal stability derived from silica-modified alumina aerogel. Catal. Lett. 58, 89–92 (1999)
A.W. Espie, J.C. Vickerman, Aluminas modified with silica. Part 1. An X-ray diffraction and secondary-ion mass spectrometry study of the influence of preparation and thermal treatment on structure and surface composition. J. Chem. Soc. Faraday Trans. 80, 1903–1913 (1984)
J. Klein, W.F. Maier, Thermal stability of sol–gel-derived porous AM-AlxZr mixed oxides. Chem. Mater. 11, 2584–2593 (1999)
T. Osaki, K. Nagashima, K. Watari, K. Tajiri, Silica-doped alumina cryogels with high thermal stability. J. Non-Cryst. Solids 353, 2436–2442 (2007)
J.-H. Lee, S.-C. Choi, D.-S. Bae, K.-S. Han, Synthesis and microstructure of silica-doped alumina composite membrane by sol–gel process. J. Mater. Sci. Lett. 18, 1367–1369 (1999)
G. Lopez-Granada, J.D.O. Barceinas-Sanchez, R. Lopez, R. Gomez, High temperature stability of anatase in titania-alumina semiconductors with enhanced photodegradation of 2,4-dichlorophenoxyacetic acid. J. Hazard. Mater. 263, 84–92 (2013)
X. Jiang, B.P. Bastakoti, W. Weng, T. Higuchi, H. Oveisi, N. Suzuki, W.-J. Chen, Y.-T. Huang, Y. Yamauchi, Preparation of ordered mesoporous alumina-doped titania films with high thermal stability and their application to high-speed passive-matrix electrochromic displays. Chem. Eur. J. 19, 10958–10964 (2013)
T. Horiuchi, Y. Teshima, T. Osaki, T. Sugiyama, K. Suzuki, T. Mori, Improvement of thermal stability of alumina by addition of zirconia. Catal. Lett. 62, 107–111 (1999)
R.H.R. Castro, S.V. Ushakov, L. Gengembre, D. Gouvea, A. Navrotsky, Surface energy and thermodynamic stability of gamma-alumina: effect of dopants and water. Chem. Mater. 18, 1867–1872 (2006)
Z. Cai, J. Li, K. Liew, J. Hu, Effect of La2O3-dopping on the Al2O3 supported cobalt catalyst for Fischer–Tropsch synthesis. J. Mol. Catal. A Chem. 330, 10–17 (2010)
C.L. Carnes, P.N. Kapoor, K.J. Klabunde, J. Bonevich, Synthesis, characterization, and adsorption studies of nanocrystalline aluminum oxide and a bimetallic nanocrystalline aluminum oxide/magnesium oxide. Chem. Mater. 14, 2922–2929 (2002)
M. Schoeneborn, A. Paeger, R. Gloeckler, Ceria zirconia alumina composition with enhanced thermal stability (Sasol Germany GmbH, Germany). Application: DE, 2013
K. Schermanz, A. Sagar, M. Schoeneborn, R. Gloeckler, K. Dallmann, F. Alber, S. Rolfs, Ceria zirconia alumina composition with enhanced thermal stability (Treibacher Industrie AG, Austria; Sasol Germany GmbH). Application: WO, 2013
R.J. Nozemack, J.F. Porinchak, Alumina-silica cogel (W. R. Grace&Co, Newyork, NY, 1988)
H.K.C. Timken, Homogeneous Modified-Alumina Fischer–Tropsch Catalyst Supports (Chevron U.S.A. Inc., USA). Application: US, 2005
C.Z. Wan, J.C. Dettling, Stabilized Alumina Catalyst Support Coatings (Engelhard Corp., USA). Application: EP, 1986
T. Fukui, M. Hori, Control of micropore size distribution in alumina by the hydrothermal treatment of an alkoxide derived-alcogel. J. Mater. Sci. 31, 3245–3248 (1996)
X. Zhao, Y. Cong, Y. Huang, S. Liu, X. Wang, T. Zhang, Rhodium supported on silica-stabilized alumina for catalytic decomposition of N2O. Catal. Lett. 141, 128–135 (2011)
T. Horiuchi, T. Osaki, T. Sugiyama, K. Suzuki, T. Mori, Maintenance of large surface area of alumina heated at elevated temperatures above 1300 °C by preparing silica-containing pseudoboehmite aerogel. J. Non-Cryst. Solids 291, 187–198 (2001)
M.K. Mardkhe, B.F. Woodfield, C.H. Bartholomew, A method of producing thermally stable and high surface area Al 2 O 3 catalyst supports (Brigham Young University, 2013)
B. Huang, C.H. Bartholomew, B.F. Woodfield, Facile synthesis of mesoporous γ-alumina with tunable pore size: the effects of water to aluminum molar ratio in hydrolysis of aluminum alkoxides. Microporous Mesoporous Mater. 183, 37–47 (2014)
A.L. Patterson, The Scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 (1939)
B. Huang, C.H. Bartholomew, B.F. Woodfield, Improved calculations of pore size distribution for relatively large, irregular slit-shaped mesopore structure. Microporous mesoporous Mater. 184, 112–121 (2014)
B. Bureau, G. Silly, J.Y. Buzaré, C. Legein, D. Massiot, From crystalline to glassy gallium fluoride materials: an NMR study of 69Ga and 71Ga quadrupolar nuclei. Solid State NMR 14, 191–202 (1999)
D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.O. Durand, B. Bujoli, Z. Gan, G. Hoatson, Modelling one and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 40, 70–76 (2002)
D.R. Neuville, L. Cormier, D. Massiot, Al environment in tectosilicate and peraluminous glasses: A 27Al MQ-MAS NMR, Raman, and XANES investigation. Geochim. Cosmochim. Acta 68, 5071–5079 (2004)
H. Yong, D. Coster, F.R. Chen, J.G. Davis, J.J. Fripiat, Aluminum coordination and Lewis acidity in aluminas and steamed zeolites. Stud. Surf. Sci. Catal. 75, 1159–1170 (1993)
W.E.E. Stone, G.M.S. El Shafei, J. Sanz, S.A. Selim, Association of soluble aluminum ionic species with a silica-gel surface: a solid-state NMR study. J. Phys. Chem. 97, 10127–10132 (1993)
L.M. Bronstein, D.M. Chernyshov, R. Karlinsey, J.W. Zwanziger, V.G. Matveeva, E.M. Sulman, G.N. Demidenko, H.-P. Hentze, M. Antonietti, Mesoporous alumina and aluminosilica with Pd and Pt nanoparticles: structure and catalytic properties. Chem. Mater. 15, 2623–2631 (2003)
R.X. Fischer, H. Schneider, M. Schmucker, Crystal structure of Al-rich mullite. Am. Mineral. 79, 983–990 (1994)
P. Burtin, J.P. Brunelle, M. Pijolat, M. Soustelle, Influence of surface area and additives on the thermal stability of transition alumina catalyst supports. II. Kinetic model and interpretation. Appl. Catal. 34, 239–254 (1987)
T. Mori, T. Horiuchi, T. Iga, Y. Murase, Large surface area of silica-coated fibrous crystals of alumina after heating at >1573 K. J. Mater. Chem. 2, 577–578 (1992)
J.S. Smith, The synthesis and structural characterization of metal oxide nanoparticles having catalytic applications (Brigham Young University, 2012)
E.J.M. Hensen, D.G. Poduval, P.C.M.M. Magusin, A.E. Coumans, J.A.R. van Veen, Formation of acid sites in amorphous silica–alumina. J. Catal. 269, 201–218 (2010)
L.L.L. Prado, P.A.P. Nascente, S.C. De Castro, Y. Gushikem, Aluminium oxide grafted on silica gel surface: study of the thermal stability, structure and surface acidity. J. Mater. Sci. 35, 449–453 (2000)
A.K. Chakraborty, Range of solid solutions of silica in spinel type phase. Adv. Appl. Ceram. 105, 297–303 (2006)
W. Daniell, U. Schubert, R. Glockler, A. Meyer, K. Noweck, H. Knozinger, Enhanced surface acidity in mixed alumina-silicas: a low-temperature FTIR study. Appl. Catal. A 196, 247–260 (2000)
M.K. Mardkhe, K. Keyvanloo, C.H. Bartholomew, W.C. Hecker, T.M. Alam, B.F. Woodfield, Acid site properties of thermally stable, silica-doped alumina as a function of silica/alumina ratio and calcination temperature. Appl. Catal. A 482, 16–23 (2014)
K. Keyvanloo, M.K. Mardkhe, T.M. Alam, C.H. Bartholomew, B.F. Woodfield, W.C. Hecker, Supported iron Fischer–Tropsch catalyst: superior activity and stability using a thermally stable silica-doped alumina support. ACS. Catal. 4, 1071–1077 (2014)
Acknowledgments
This work was supported by the U.S. Department of Energy under grant DE-FG02-05ER15666 and National Science Foundation under CHE-0959862. The solid state NMR (TMA) was performed at Sandia National Laboratories which is a multi-program laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-AC04-94AL85000. We also thank Dr. Jeff Farrer and the BYU microscopy lab for their assistance with the TEM imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mardkhe, M.K., Huang, B., Bartholomew, C.H. et al. Synthesis and characterization of silica doped alumina catalyst support with superior thermal stability and unique pore properties. J Porous Mater 23, 475–487 (2016). https://doi.org/10.1007/s10934-015-0101-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-0101-z