Abstract
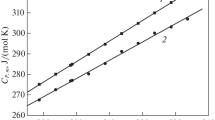
This paper reports on our mass spectrometric study of sublimation of glycine and DL-alanyl-glycine (Ala-gly). The sublimation enthalpy of Ala-gly has been determined by generalization of the data obtained and the results of AM1 quantum-chemical calculations. A relationship has been found between the sublimation enthalpy (ΔH subl), heat capacity (C P), and the sum of bond lengths (Σn i l i ) in 17 α-amino acid and 9 dipeptide molecules. Correlations are suggested for evaluating ΔH subl of amino acids and peptides.
Similar content being viewed by others
References
J. Campbell, Modern General Chemistry [Russian translation], Vol. 3, Mir, Moscow (1975).
A. M. Kutepov (ed.), Biologically Active Substances in Solutions: Structure, Thermodynamics, Reactivity [in Russian], Nauka, Moscow (2001).
A. Kvick, Acta Crystallogr., B33, 3796–3801 (1977).
R. Destro, R. Bianchi, and G. Morosi, J. Phys. Chem., 93, 4447–4457 (1989).
Ph. Coppens, Yu. Abramov, M. Carducci, et al., J. Am. Chem. Soc., 121, 2585–2593 (1999).
H. Ratajczak and W. J. Orville-Thomas, Molecular Interactions, Wiley, New York (1980).
V. Kalous and Z. Pavlicek, Biophysical Chemistry, Freeman, San Francisco (1980).
C. G. De Kruif, J. Voogd, and J. C. A. Offringa, J. Chem. Thermodyn., 11, 651–656 (1979).
T. Cotterell, Strength of the Chemical Bond [Russian translation], Inostrannaya Literatura, Moscow (1956).
E. A. Arnautova, M. V. Zakhrova, T. S. Pivina, et al., Izv. Ross. Akad. Nauk, Ser. Khim., No. 12, 2872–2881 (1996).
S. Bradschneider, The Properties of Gases and Liquids [in Russian], Ch. V, Khimiya, Moscow (1966), pp. 162–165.
V. S. Vatagin and V. G. Badelin, Zh. Khim. Termodin. Termokhim., 2, No. 1, 101–105 (1993).
Yu. A. Lebedev and E. A. Miroshnichenko, Thermochemistry of Vaporization of Organic Substances [in Russian], Nauka, Moscow (1981).
S. Nagaki, H. Chihara, and S. Seki, Bull. Chem. Soc. Jpn., 32, 84–88 (1959).
S. Ngauv, R. Sabbah, and M. Laffite, Termochim. Acta, 20, 371–374 (1977).
H. J. Svec and D. D. Clyde, J. Chem. Eng. Data, 10, 151/152 (1965).
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart, J. Am. Chem. Soc., 107, No. 13, 3902–3909 (1985).
A. I. Kitaigorodskii, P. M. Zorkii, and V. K. Belskii, Structure of Organic Substances. Structural Data [in Russian], Nauka, Moscow (1980).
A. E. Smolyar, A. R. Abramov, O. A. Narimbekov, and T. N. Shakhtinskii, Azerb. Khim. Zh., No. 2, 42–47 (1984).
R. Flaiy, T. Koritsanszky, D. Zobel, and P. Luger, J. Am. Chem. Soc., 120, 2227–2238 (1998).
M. Souhassou, C. Lecomte, N. E. Ghermani, et al., ibid., 114, 2371–2382 (1992).
V. V. Ponomarev, Zh. Fiz. Khim., 36, 1472–1476 (1962).
J. C. R. Reis, M. J. Blandamer, M. I. Davis, and G. Douheret, Phys. Chem. Chem. Phys., 3, 1465–1470 (2001).
R. Sabban and M. Laffitti, Bull. Soc. Chem. France, 1, 50–52 (1978).
G. C. Barret (ed.), Chemistry and Biochemistry of the Amino Acids, Chapman and Hall, London (1985).
V. S. Kuzmin and S. B. Kazer, Izv. Ross. Akad. Nauk, Ser. Khim., No. 4, 922–931 (1992).
V. G. Badelin, O. V. Kulikov, V. S. Vatagin, et al., Thermochim. Acta, 169, 81–93 (1990).
N. M. Bazhin, V. A. Ivanchenko, and V. N. Parmon, Thermodynamics for Chemists [in Russian], Khimiya, Moscow (2001).
E. J. Cohn, T. L. McMeekin, J. D. Ferry, and M. H. Blanchard, J. Phys. Chem., 43, 169 (1939).
B. Honig and A. S. Yang, Adv. Protein Chem., 46, 27 (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 48, No. 4, pp. 698–704, July–August, 2007.
Original Russian Text Copyright © 2007 by V. G. Badelin, E. Yu. Tyunina, G. V. Girichev, N. I. Giricheva, and O. V. Pelipets
Rights and permissions
About this article
Cite this article
Badelin, V.G., Tyunina, E.Y., Girichev, G.V. et al. Relationship between the molecular structure of amino acids and dipeptides and thermal sublimation effects. J Struct Chem 48, 647–653 (2007). https://doi.org/10.1007/s10947-007-0098-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-007-0098-5