Abstract
The effects of various cations (Li+, Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Sr2+, Ba2+, Mn2+, Co2+, and Ni2+) and anions (Cl−, Br−, I−, \( {\text{NO}}_{3}^{ - } \), \( {\text{ClO}}_{4}^{ - } \), \( {\text{HCO}}_{3}^{ - } \), and \( {\text{CO}}_{3}^{2 - } \)) on the molar absorptivity of water in the OH stretching band region (2,600–3,800 cm−1) were ascertained from attenuated total reflection infrared spectra of aqueous electrolyte solutions (22 in all). The OH stretching band mainly changes linearly with ion concentrations up to 2 mol·L−1, but several specific combinations of cations and anions (Cs2SO4, Li2SO4, and MgSO4) present different trends. That deviation is attributed to ion pair formation and cooperativity in ion hydration, which indicates that the extent of the ion–water interaction reflected by the OH stretching band of water is beyond the first solvation shell of water molecules directly surrounding the ion. The obtained dataset was then correlated with several quantitative parameters representing structural and dynamic properties of water molecules around ions: ΔG HB, the structural entropy (S str), the viscosity B-coefficient (B η ), and the ionic B-coefficient of NMR relaxation (B NMR). Results show that modification of the OH stretching band of water caused by ions has quasi-linear relations with all of these parameters. Vibrational spectroscopy can be a useful means for evaluating ion–water interaction in aqueous solutions.






Similar content being viewed by others
References
Masuda, K., Haramaki, T., Nakashima, S., Habert, B., Martinez, I., Kashiwabara, S.: Structural change of water with solutes and temperature up to 100 °C in aqueous solutions as revealed by attenuated total reflectance infrared spectroscopy. Appl. Spectrosc. 57, 274–281 (2003)
Li, R.H., Jiang, Z.P., Shi, S.Q., Yang, H.W.: Raman spectra and O-17 NMR study effects of CaCl2 and MgCl2 on water structure. J. Mol. Struct. 645, 69–75 (2003)
Li, R.H., Jiang, Z.P., Chen, F.G., Yang, H.W., Guan, Y.T.: Hydrogen bonded structure of water and aqueous solutions of sodium halides: a Raman spectroscopic study. J. Mol. Struct. 707, 83–88 (2004)
Li, R.H., Jiang, Z.P., Guan, Y.T., Yang, H.W., Liu, B.: Effects of metal ion on the water structure studied by the Raman O–H stretching spectrum. J. Raman Spectrosc. 40, 1200–1204 (2009)
Chen, Y., Zhang, Y.H., Zhao, L.J.: ATR-FTIR spectroscopic studies on aqueous LiClO4, NaClO4, and Mg(ClO4)2 solutions. Phys. Chem. Chem. Phys. 6, 537–542 (2004)
Wei, Z.F., Zhang, Y.H., Zhao, L.J., Liu, J.H., Li, X.H.: Observation of the first hydration layer of isolated cations and anions through the FTIR-ATR difference spectra. J. Phys. Chem. A 109, 1337–1342 (2005)
Liu, J.H., Zhang, Y.H., Wang, L.Y., Wei, Z.F.: Drawing out the structural information of the first layer of hydrated ions: ATR-FTIR spectroscopic studies on aqueous NH4NO3, NaNO3, and Mg(NO3)2 solutions. Spectrochim. Acta Part A 61, 893–899 (2005)
Zhao, L.J., Zhang, Y.H., Wei, Z.F., Cheng, H., Li, X.H.: Magnesium sulfate aerosols studied by FTIR spectroscopy: hygroscopic properties, supersaturated structures, and implications for seawater aerosols. J. Phys. Chem. A 110, 951–958 (2006)
Dong, J.L., Li, X.H., Zhao, L.J., Xiao, H.S., Wang, F., Guo, X., Zhang, Y.H.: Raman observation of the interactions between \( {\text{NH}}_{4}^{ + } \), \( {\text{SO}}_{4}^{2 - } \), and H2O in supersaturated (NH4)2SO4 droplets. J. Phys Chem. B 111, 12170–12176 (2007)
Kataoka, Y., Kitadai, N., Hisatomi, O., Nakashima, S.: Nature of hydrogen bonding of water molecules in aqueous solutions of glycerol by attenuated total reflection (ATR) infrared spectroscopy. Appl. Spectrosc. 65, 436–441 (2011)
Sun, Q.: Raman spectroscopic study of the effects of dissolved NaCl on water structure. Vib. Spectrosc. 62, 110–114 (2012)
Guo, Y.C., Li, X.H., Zhao, L.J., Zhang, Y.H.: Drawing out the structural information about the first hydration layer of the isolated Cl− anion through the FTIR-ATR difference spectra. J. Solution Chem. 42, 459–469 (2013)
Walrafen, G.E., Hokmabadi, M.S., Yang, W.H.: Raman isosbestic points from liquid water. J. Chem. Phys. 85, 6964–6969 (1986)
Terpstra, P., Combes, D., Zwick, A.: Effect of salts on dynamics of water—a Raman-spectroscopy study. J. Chem. Phys. 92, 65–70 (1990)
Pastorczak, M., Kozanecki, M., Ulanski, J.: Raman resonance effect in liquid water. J. Phys. Chem. A 112, 10705–10707 (2008)
Hancer, M., Sperline, R.P., Miller, J.D.: Anomalous dispersion effects in the IR-ATR spectroscopy of water. Appl. Spectrosc. 54, 138–143 (2000)
Walrafen, G.E.: Raman spectral studies of effects of solutions and pressure on water structure. J. Chem. Phys. 55, 768–792 (1971)
Bertie, J.E., Eysel, H.H.: Infrared intensities of liquids. 1. Determination of infrared optical and dielectric-constants by FT-IR using the circle ATR cell. Appl. Spectrosc. 39, 392–401 (1985)
Weast, C.: Handbook of Chemistry and Physics, 57th edn. CRC Press, Cleveland (1977)
Li, H.H.: Reflective-index of ZnS, ZnSe, and ZnTe and its wavelength and temperature derivatives. J. Phys. Chem. Ref. Data 13, 103–150 (1984)
Bertie, J.E., Lan, Z.: Infrared intensities of liquids. 20. The intensity of the OH stretching band of liquid water revisited, and the best current values of the optical constants of H2O(l) at 25 °C between 15,000 and 1 cm−1. Appl. Spectrosc. 50, 1047–1057 (1996)
Keefe, C.D., Wilcox, T., Campbell, E.: Measurement and applications of absolute infrared intensities. J. Mol. Struct. 1009, 111–122 (2012)
Ayerst, R.P., Phillips, M.I.: Solubility and refractive index of ammonium perchlorate in water. J. Chem. Eng. Data 11, 494–496 (1966)
Yu, X., Zeng, Y., Yao, H., Yang, J.: Metastable phase equilibria in the aqueous ternary systems KCl + MgCl2 + H2O and KCl + RbCl + H2O at 298.15 K. J. Chem. Eng. Data 56, 3384–3391 (2011)
Max, J.J., Chapados, C.: Infrared spectra of cesium chloride aqueous solutions. J. Chem. Phys. 113, 6803–6814 (2000)
Max, J.J., Chapados, C.: IR spectroscopy of aqueous alkali halide solutions: pure salt-solvated water spectra and hydration numbers. J. Chem. Phys. 115, 2664–2675 (2001)
Max, J.J., de Blois, S., Veilleux, A., Chapados, C.: IR spectroscopy of aqueous alkali halides. Factor analysis. Can. J. Chem. 79, 13–21 (2001)
Urrejola, S., Sanchez, A., Hervello, M.F.: Refractive indices of lithium, magnesium, and copper(II) sulfates in ethanol–water solutions. J. Chem. Eng. Data 55, 482–487 (2010)
Urrejola, S., Sanchez, A., Hervello, M.F.: Refractive indices of sodium, potassium, and ammonium sulfates in ethanol water solutions. J. Chem. Eng. Data 55, 2924–2929 (2010)
Seidell, A.: Solubilities of Inorganic and Organic Compounds: A Compilation of Quantitative Solubility Data from the Periodical Literature, 2nd edn. Nostrand, New York (1919)
Rudolph, W.W., Irmer, G., Hefter, G.T.: Raman spectroscopic investigation of speciation in MgSO4(aq). Phys. Chem. Chem. Phys. 5, 5253–5261 (2003)
Buchner, R., Chen, T., Hefter, G.: Complexity in “simple” electrolyte solutions: ion pairing in MgSO4(aq). J. Phys. Chem. B 108, 2365–2375 (2004)
Hefter, G.: When spectroscopy fails: the measurement of ion pairing. Pure Appl. Chem. 78, 1571–1586 (2006)
Larentzos, J.P., Criscenti, L.J.: A molecular dynamics study of alkaline earth metal-chloride complexation in aqueous solution. J. Phys. Chem. B 112, 14243–14250 (2008)
Callahan, K.M., Casillas-Ituarte, N.N., Roeselova, M., Allen, H.C., Tobias, D.J.: Solvation of magnesium dication: molecular dynamics simulation and vibrational spectroscopic study of magnesium chloride in aqueous solutions. J. Phys. Chem. A 114, 5141–5148 (2010)
Wachter, W., Fernandez, S., Buchner, R., Hefter, G.: Ion association and hydration in aqueous solutions of LiCl and Li2SO4 by dielectric spectroscopy. J. Phys. Chem. B 111, 9010–9017 (2007)
Tielrooij, K.J., Garcia-Araez, N., Boon, M., Bakker, H.J.: Cooperativity in ion hydration. Science 328, 1006–1009 (2010)
Smith, J.D., Cappa, C.D., Wilson, K.R., Cohen, R.C., Geissler, P.L., Saykally, R.J.: Unified description of temperature-dependent hydrogen-bond rearrangements in liquid water. Proc. Natl. Acad. Sci. USA 102, 14171–14174 (2005)
Eaves, J.D., Loparo, J.J., Fecko, C.J., Roberts, S.T., Tokmakoff, T., Geissler, P.L.: Hydrogen bonds in liquid water are broken only fleetingly. Proc. Natl. Acad. Sci. USA 102, 13019–13022 (2005)
Bakker, H.J., Skinner, J.L.: Vibrational spectroscopy as a probe of structure and dynamics of liquid water. Chem. Rev. 110, 1498–1517 (2010)
Marcus, Y.: Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 109, 1346–1370 (2009)
Marcus, Y.: Viscosity B-coefficients, structural entropies and heat-capacities, and the effects of ions on the structure of water. J. Solution Chem. 23, 831–848 (1994)
Engel, G., Hertz, H.G.: On negative hydration. A nuclear magnetic relaxation study. Ber. Bunsenges. Phys. Chem. 72, 808–834 (1968)
Max, J.J., Chapados, C.: Influence of anomalous dispersion on the ATR spectra of aqueous solutions. Appl. Spectrosc. 53, 1045–1053 (1999)
Acknowledgments
We greatly appreciate Dr. Tadashi Yokoyama and Mr. Naoki Nishiyama of Osaka University for their help with sample preparations. We also thank two anonymous referees and associated editor Luigi Paduano for their careful reviews of this manuscript. This research was financially supported by a JSPS Research Fellowship for Young Scientists to Norio Kitadai.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
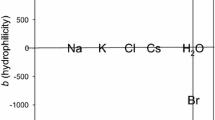
Although it is not the main subject of this study, we show the effects of 1 mol·L−1 cations and 1 mol·L−1 anions on the molar absorptivity of water in the OH bending band region (1,200–2,000 cm−1) in Fig. 7. This presentation of the influences of various ions on the OH bending band of water is the first ever reported. We hope that these results can stimulate future theoretical and/or experimental studies in this area.
Effects of 1 mol·L−1 cations (a) and 1 mol·L−1 anions (b) on the molar absorptivity of water in the OH bending band region (1,200–2,000 cm−1). The effect of \( {\text{HCO}}_{3}^{ - } \) is not shown in this figure because of the strong overlap with a \( {\text{HCO}}_{3}^{ - } \) band [1]. The OH bending band of water is shown at the top of each figure for comparison
We also present in Tables 2 and 3 a summary of the aqueous solutions measured in this study, and the numerical results on the areas of ∆MAL–H and ∆ATRL–H for each ion (at 1 mol·L−1 concentration), respectively.
Rights and permissions
About this article
Cite this article
Kitadai, N., Sawai, T., Tonoue, R. et al. Effects of Ions on the OH Stretching Band of Water as Revealed by ATR-IR Spectroscopy. J Solution Chem 43, 1055–1077 (2014). https://doi.org/10.1007/s10953-014-0193-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0193-0