Abstract
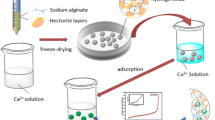
Granular alginate-based hydrogels were prepared in situ in an aqueous solution via grafting and crosslinking reactions among sodium alginate (SA), acrylic acid (AA), polyvinylpyrrolidone (PVP), and gelatin (GE). Fourier transform infrared spectra, elemental analysis, and scanning electrical microscopy revealed that AA monomers were grafted onto an SA backbone, and that PVP and GE were present in the hydrogel network as linear interpenetrating components. The grafting polymerization and crosslinking reaction between only SA and AA yielded a bulk gel, but the introduction of PVP and GE into the reaction mixture led to the formation of granular products. Electrostatic and hydrogen-bonding interactions among SA, PAA, PVP, and GE were the main driving forces for the formation of granular products. The adsorption isotherms and adsorption kinetics were evaluated for the adsorption of model heavy-metal ions on one of the hydrogels. The results indicated that the hydrogel has satisfactory adsorption capacities (3.028 mmol/g, Ni2+; 3.146 mmol/g, Cu2+; 2.911 mmol/g, Zn2+; 2.862 mmol/g, Cd2+), adsorption rates, and recovery capacities for the target metal ions. In addition, competitive adsorption results suggested that the hydrogel has a stronger affinity for Cu2+ ion than for the other ions.








Similar content being viewed by others
References
Nakason C, Wohmang T, Kaesaman A, Kiatkamjornwong S (2010) Carbohydr Polym 81:348–357
Marandi GB, Mahdavinia GR, Ghafary S (2011) J Polym Res 18:1487–1499
Al E, Güçlü G, İyim TB, Emik S, Özgümüş S (2008) J Appl Polym Sci 109:16–22
Wang WB, Wang AQ (2010) Carbohydr Polym 82:83–91
Kevadiya BD, Joshi GV, Mody HM, Bajaj HC (2011) Appl Clay Sci 52:364–367
Ngadaonye JI, Cloonan MO, Geever LM, Higginbotham CL (2011) J Polym Res 18:2307–2324
Güçlü G, Al E, Emik S, İyim TB, Özgümüş S, Özyürek M (2009) Polym Bull 65:333–346
Guilherme MR, Reis AV, Paulino AT, Fajardo AR, Muniz EC, Tambourgi EB (2007) J Appl Polym Sci 105:2903–2909
Milosavljević NB, Ristić MĐ, Perić-Grujić AA, Filipović JM, Štrbac SB, Rakočević ZLJ, Krušić MTK (2011) Colloids Surf A 388:59–69
Kangwansupamonkon W, Jitbunpot W, Kiatkamjornwong S (2010) Polym Deg Stab 95:1894–1902
Léger B, Menuel S, Ponchel A, Hapiot F, Monflier E (2012) Adv Synth Catal 354:1269–1272
Saikia AK, Mandal UK, Aggarwal S (2012) J Polym Res 19:9871
Román J, Cabañas MV, Peña J, Vallet-Regí M (2011) Sci Technol Adv Mater 12:045003
Zhu JM (2010) Biomaterials 31:4639–4656
Sud D, Mahajan G, Kaur MP (2008) Bioresource Technol 99:6017–6027
Demirbas A (2008) J Hazard Mater 157:220–229
Liu Y, Wang WB, Wang AQ (2010) Desalination 259:258–264
Zheng YA, Hua SB, Wang AQ (2010) Desalination 263:170–175
Şolpan D, Duran S, Saraydin D, Güven O (2003) Rad Phys Chem 66:117–127
Chen H, Wang AQ (2009) J Hazard Mater 165:223–231
Pekel N, Güven O (2003) Colloids Surf A 212:155–161
Çaykara T, İnam R (2003) J Appl Polym Sci 89:2013–2018
Song F, Tang DL, Wang XL, Wang YZ (2011) Biomacromoles 12:3369–3380
Avérous L, Pollet E (2012) Biodegradable polymers. In: Environmental silicate nano-biocomposites. Springer, London, pp 13–39
Ray SS, Bousmina M (2005) Prog Mater Sci 50:962–1079
Stewart TJ, Yau JH, Allen MM, Brabander DJ, Flynn NT (2009) Colloid Polym Sci 287:1033–1040
Sand A, Yadav M, Mishra DK, Behari K (2010) Carbohydr Polym 80:1147–1154
Yadav M, Mishra DK, Sand A, Behari K (2011) Carbohydr Polym 84:83–89
Helwa Y, Dave N, Froidevaux R, Samadi A, Liu JW (2012) ACS Appl Mater Interf 4:2228–2233
Liu MZ, Liang R, Zhan FL, Liu Z, Niu AZ (2007) Polym Int 56:729–737
Hua Y, Jiang XQ, Ding Y, Ge HX, Yuan YY, Yang CZ (2002) Biomaterials 23:3193–3201
Xie YT, Wang AQ (2009) J Polym Res 16:143–150
Robinson BV, Sullivan FM, Borzelleca JF, Schwartz SL (1990) PVP: a critical review of the kinetics and toxicology of polyvinylpyrrolidone (Povidone), 1st edn. Lewis, Chelsea
Hu YG, Zhao T, Zhu PL, Sun R (2012) Colloid Polym Sci 290:401–409
Pourjavadi A, Farhadpour B, Seidi F (2008) Starch-Starke 60:457–466
Jin SP, Liu MZ, Zhang F, Chen SL, Niu AZ (2006) Polymer 47:1526–1532
Žugić D, Spasojević P, Petrović Z, Djonlagić J (2009) J Appl Polym Sci 113:1593–1603
Jaiswal M, Koul V, Dinda AK, Mohanty S, Jain KG (2011) J Biomed Mater Res B 98B:342–350
Langmuir I (1918) J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Z Phys Chem 57:385–470
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Proc Acad Sci USSR 55:331–333
Rudzinsk W, Plazinski W (2006) J Phys Chem B 110:16514–16525
Acknowledgment
This work is supported by the National Natural Science Foundation of China (nos. 51003112 and 21107116)) and the Science and Technology Support Project of Jiangsu Provincial Sci. & Tech. Department (no. BY2010012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Kang, Y. & Wang, A. One-step fabrication in aqueous solution of a granular alginate-based hydrogel for fast and efficient removal of heavy metal ions. J Polym Res 20, 101 (2013). https://doi.org/10.1007/s10965-013-0101-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0101-0