Abstract
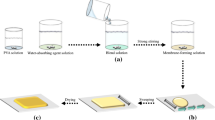
In the present study, integrated polymers having gas barrier and water sorption properties were prepared by effectively blending two types of polymers to fabricate membranes for high-performance CO2 separation. Namely, composite polymers were prepared as a new membrane material by uniformly blending polyvinyl alcohol (PVA), a gas barrier polymer, with sodium polyacrylate (PAANa), a water-absorbing polymer. The optimal PVA/PAANa blending ratio was determined by evaluating the thermal properties of the prepared polymer blend and the CO2 separation performance of the polymer blend membrane. When amine type additives such as polyamidoamine (PAMAM) dendrimer or polyallylamine (PAAm) were added to the prepared PVA/PAANa, the separation performance of the produced separation membrane increased. Applying a carbonate coating onto the PVA/PAANa membranes containing additives further increased the separation performance and selectivity of the membranes. These results demonstrated that the PVA/PAANa membranes prepared in this study, which featured both gas barrier and water absorption properties, could be used as high-performance membrane materials for CO2 separation.









Similar content being viewed by others
References
Duan S, Kouketsu T, Kazama S, Yamada K (2006) Development of PAMAM dendrimer composite membranes for CO2 separation. J Membrane Sci 283:2–6
Minelli M, Medri V, Papa E, Miccio F, Landi E (2016) Geopolymers as solid adsorbent for CO2 capture. Chemical Eng Sci 148:267–274
Muchan P, Saiwan C, Narku-Tetteh J, Idem R, Supap T, Tontiwachwuthikui P (2016) Screening tests of aqueous alkanolamine solutions based on primary, secondary, and tertiary structure for blended aqueous amine solution selection in post combustion CO2 capture. Chemical Eng Sci 170:574–582
Kai T, Taniguchi I, Duan S, Chowdhury FA, Saito T, Yamazaki K, Ikeda K, Ohara T, Asano S, Kazama S (2013) Molecular gate membrane: poly (amidoamine) dendrimer/polymer hybrid membrane modules for CO2 capture. Energy Procedia 37:961–968
Duan S, Taniguchi I, Kai T, Kazama S (2012) Poly (amidoamine) dendrimer/poly (vinyl alcohol) hybrid membranes for CO2 capture. J Membrane Sci 423–424:107–112
Duan S, Taniguchi I, Kai T, Kazama S (2013) Development of poly (amidoamine) dendrimer/polyvinyl alcohol hybrid membranes for CO2 capture at elevated pressures. Energy Procedia 37:924–931
Duan S, Chowdhury FA, Kai T, Kazama S, Fujioka Y (2008) PAMAM dendrimer composite membrane for CO2 separation: addition of hyaluronic acid in gutter layer and application of novel hydroxyl PAMAM dendrimer. Desalination 234:278–285
Car A, Stropnik C, Yave W, Peinemann K-V (2008) PEG modified poly (amide-b-ethylene oxide) membranes for CO2 separation. J Membrane Sci 307:88–95
Taniguchi I, Kai T, Duan S, Kazama S, Jinnai H (2015) A compatible crosslinker for enhancement of CO2 capture of poly (amidoamine) dendrimer-containing polymeric membranes. J Membrane Sci 475:175–183
Guerrero G, Venturi D, Peters T, Rival N, Denonville C, Simon C, Henriksen PP, Hägg M-B (2017) Influence of functionalized nanoparticles on the CO2/N2 separation properties of PVA-based gas separation membranes. Energy Procedia 114:627–635
Yang H, Xu Z, Fan M, Gupta R, Slimane RB, Bland AE, Wright I (2008) Progress in carbon dioxide separation and capture: A review. J Environmental Sci 20:14–27
Olajire AA (2010) CO2 capture and separation technology for end-of-pipe applications – a review. Energy 35:2610–2628
D’Alesssandro DM, Smit B, Long JR (2010) Carbon dioxide capture: prospects for new materials. Angew Chem 49:6058–6082
Brunetti A, Scura F, Barbieri G, Drioli E (2010) Membrane technologies for CO2 separation. J Membrane Sci 359:115–125
Luis P, Gerven TV, Bruggen BVD (2012) Recent developments in membrane-based technologies for CO2 capture. Prog Energy Combust Sci 38:419–448
Kouketsu T, Duan S, Kai T, Kazama S, Yamada K (2007) PAMAM dendrimer composite membrane for CO2 separation: formation of a chitosan gutter layer. J Membrane Sci 287:51–59
Duan S, Kai T, Taniguchi I, Kazama S (2014) Development of poly (amidoamine) dendrimer/poly(ethyleneglycol) hybrid membranes for CO2 capture at elevated pressures. Energy Procedia 63:167–173
Duan S, Kai T, Saito T, Yamazaki K, Ikeda K (2014) Effect of cross-linking on the mechanical and thermal properties of poly(amidoamine) dendrimer/poly(vinyl alcohol) hybrid membranes for CO2 separation. Membranes 4:200–209
Yegani R, Hirozawa H, Teramoto M, Himei H, Okada O, Takigawa T, Ohmura N, Matsumiya N, Matsuyama H (2007) Selective separation of CO2 by using novel facilitated transport membrane at elevated temperature and pressures. J Membrane Sci 291:157–164
Uemoto T, Sugiura K, Okada O, Nonouchi T, Ito F, Akiyama K, Matsuda K (2013) Proposition of CO2 removable technology using membrane for Hydrogen Station. ECS Trans 51(1):259–264
Carrera MC, Erdmann E, Destéfanis HA (2013) Barrier properties and structural study of nanocomposite of HDPE/montmorillonite modified with Polyvinylalcohol. J Chem https://doi.org/10.1155/2013/679567
Cui Y, Kumar S, Kona BR, Houcke DV (2015) Gas barrier properties of polymer/clay nanocomposites. RSC Adv 5:63669–63690
Adewole JK, Ahmad AL (2017) Polymeric membrane materials selection for high-pressure CO2 removal from natural gas. J Polym Res 24:70–82
Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant JP17K00634.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, F., Nishiyama, Y., Duan, S. et al. Development of high-performance polymer membranes for CO2 separation by combining functionalities of polyvinyl alcohol (PVA) and sodium polyacrylate (PAANa). J Polym Res 26, 106 (2019). https://doi.org/10.1007/s10965-019-1769-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1769-6