Abstract
The activity concentrations of 234U and 238U in thermal groundwater, deep well water and river water samples from Central Poland were determined. Concentration of 234U and 238U in the examined waters varied from <0.013 (LLD) to 16.8 mBq/dm3 and from <0.013 (LLD) to 45.5 mBq/dm3 respectively. The highest uranium activity concentrations were measured in the thermal groundwater from Mszczonow and Cieplice, while the lowest were observed in thermal ground water from Uniejow and Poddebice. In thermal groundwater from Skierniewice, uranium activity concentrations were below lower limit of detection (0.013 mBq/dm3). The 234U/238U activity ratio varied from 0.37 (Cieplice) to 1.30 (Poddebice well water).
Similar content being viewed by others
Introduction
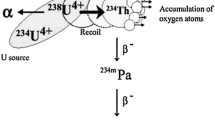
Uranium chain radionuclides are usually used for a wide range of applications in the Earth Sciences. The 234U/238U activity ratio is used as a geochemical tool to investigate transport and flow relationships in major hydrological reservoirs, groundwater pattern and it is highly useful for interpreting timescales of weathering. In rocks older than a few million years, 234U/238U activity ratios should be in secular equilibrium [1]. Many studies on 238U and 234U in natural waters indicate that these isotopes occur in a disequilibrium state and that, with a few exceptions, water samples contain higher 234U activities than 238U [2–4]. The higher activity of 234U in waters is the result of the 234U atom displacement from the crystal lattice after 238U decay and recoil of the formed 234Th nuclide, which after two consecutive decays finally form the 234U nuclide. It allows 234U to be more mobile during the weathering of rocks by atmospheric water. The recoil 234U atoms are liable to be oxidized to the hexavalent stage and can be leached into the water phase more easily than its parent nuclide 238U. The oxidation of U(IV) to U(VI) is an important step in leaching into water, because compounds of U(VI) have a higher solubility in water [2, 3, 5, 6]. The uranium isotope ratio is also influenced by other factors: the age of the rocks, rock type and climate difference [1]. This fact has been successfully applied for geological tracing or the characterization of geochemical processes.
In the same way, the 235U/238U isotope ratio (natural value 7.25 × 10−3) has been used for the identification of anthropogenic pollution scenarios [7, 8]. 235U is a key isotope in nuclear fuel manufacture and reprocessing, and any deviation from the expected natural isotope ratio in environmental samples is an indication of contamination from nuclear activity. Large increases in the 236U/238U isotopic ratio represent have been used as a clear indicator of the presence of irradiated uranium [8, 9].
Average 238U and 234U activity concentrations in surface and ground water are in the range from 1 to 20 mBq/dm3 [10]. A major source of uranium is seawater, where its average concentration of 238U equal to 41.5 mBq/dm3 [11]. The highest uranium activity concentrations were detected in the drinking water in a private well in Finland −150 Bq/dm3 [12].
The uranium activity ratio can be measured in diverse matrices by radiometric, mass spectrometry and alpha spectrometry methods. Advances in the methods of uranium activity ratio determination have been recently overviewed [13–15].
Recently we have found significant differences in the some natural radionuclide activity concentrations in deep well water samples (including thermal groundwater) originating basically from the same Lower Cretaceous geological formation [16]. Since the salinity of these samples differs also substantially one possible explanation for this phenomenon is the possible infiltration of surface water to these underground reservoirs. Special attention has been devoted to a newly exploited source of the thermal groundwater from Poddebice close to the city of Lodz. In very close vicinity of this thermal groundwater source lying 2,000 m below ground level, there are two other important water sources: an underground water reservoir (70 m below surface) used as a source of municipal drinking water, and the Ner river. The Ner river flows mostly through the rural area, and the main source of uranium in its water should be the dissolved uranium from fertilizers.
Therefore, it seems to be interesting to compare the uranium activity ratio in the selected water samples in order to detect the possible vertical transport of the surface water to the deep underground thermal water reservoir.
Experimental
Study area
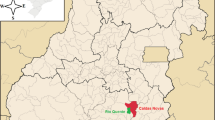
Samples of thermal groundwater, groundwater and surface water was collected from different sites from central Poland (Fig. 1).
In the case of Poddebice, the water samples were taken from three water sources:
-
(a)
The Ner river
-
(b)
Well municipal water (70 m below surface)
-
(c)
Thermal groundwater reservoir (2,000 m) as is shown in Fig. 2.
Ten litres of each water sample were collected in polyethylene bottles with screw caps. All samples were acidified to pH ≈ 2 directly after collection. As the water samples presented complete clarity, filtering of the samples was considered unnecessary.
For the Poddebice sites, several soil samples from different levels of the bore-hole were also taken for uranium radionuclides analysis.
Radiochemical method
Uranium isotopes were co-precipitated from water samples with hydrated manganese dioxide by a method described elsewhere [17–19].
Hydrated manganese dioxide was dissolved in 50 mL of 3% H2O2 in 1 M HCl solution and evaporated to dryness. The residue was dissolved in 50 mL of 9 M HCl and was passed through the anion exchangeable resin Dowex 1X8 (50–100 mesh, Cl− form). Each column was first conditioned by 50 mL 9 M HCl. Under this condition, uranium is adsorbed by the resin, whereas thorium and other elements are passed with the solution and discarded [20]. Then the column was washed with 20 mL of 9 M HCl to eliminate residual thorium traces. Cations of U+4, U+6 and Fe+3 were retained on the column. Iron ion was eluted with 25 mL of 8 M HNO3. The remaining uranium was eluted in two portion: with 25 mL of 8 M HNO3 and with 50 mL deionized water, and the total collected solution was evaporated. After evaporation the solid residue containing uranium was dissolved in 0.75 M (NH4)2SO4 solution and then it was transferred to an electrolytic cell. Uranium isotopes (234U and 238U) are electrodeposited on a stainless steel disc at a current density of 1.2 A/cm2 for 1.5 h. Fifteen minutes before ending the electrolysis, 0.5 mL NH4OH solution was added to the electrolyte to complete the uranium deposition on the disk. The disk with uranium was washed with deionized water and acetone.
The activity concentrations of uranium isotopes were determined using an α spectrometry system with PIPS detector (Canberra Packard). Lower limit of detection, for 300,000 s counting time, were equal to 0.013 mBq/dm3 for each of the uranium isotopes.
The activity concentrations of 238U via its daughter 234Th in the soil samples were determined by gamma spectrometry with HPGe detector using its principal γ-line 63.3 keV.
Quality assurance of the elaborated method
The accuracy of the developed method for uranium determination was evaluated by checking the activity concentration of 234U and 238U in two Standard Reference Materials: IAEA Soil 327, IAEA Phosphogypsum 434 and IAEA water samples distributed for intercomparison measurements—CU-2010-03. The average uranium recovery was almost quantitatively (varied from 92 to 100%). The results for intercomparison measurements—CU-2010-03 are presented in Table 1.
Results and discussion
Activity concentration of uranium isotopes in Poddebice region
The activity concentration of 234U and 238U in the water samples taken from three sources around the geothermal bore-hole of Poddebice in different months of 2011 are presented in Table 2.
The lowest activity concentration of 234U and 238U were found in the thermal groundwater samples, and varied from 0.087 to 0.168 mBq/dm3 and from 0.092 to 0.176 mBq/dm3 for 234U and 238U respectively. As expected, the highest contents of uranium isotopes were found in the river water, from the Ner, as a result of additional anthropogenic uranium input from agricultural application of phosphoric fertilizer [21]. In this water activity, concentrations varied from 1.43 to 8.54 mBq/dm3 and from 1.15 to 7.96 mBq/dm3 for 234U and 238U respectively.
The slight seasonal decrease of uranium concentration in the Ner river, connected with periods of rain, can be also observed (Fig. 3). Similar levels of 238U activity concentration and its seasonal fluctuation were observed for other Polish main rivers [21, unpublished data].
234U/238U isotopic ratio in Poddebice region
As was mentioned, the interesting information concerning mutual transfer between underground and surface water can be obtained from a comparison of 234U/238U activity ratio in these water samples. The results of the seasonal fluctuations of 234U/238U in water samples from Poddebice are shown in Fig. 4.
In thermal groundwater from Poddebice, the activity ratio of 234U/238U was the lowest with an average value 0.935 ± 0.021, and practically does not change seasonally.
In deep well water exploited from 70 m below ground level, the uranium isotope activity ratio changed from 0.9 in June to 1.30 in April.
As was mentioned, most groundwater samples have 234U/238U activity ratio above 1. An excess of 234U is explained by α-recoil of 234Th into solution and by preferential leaching of 234U from soil [22, 23]. However this phenomenon plays a role for water reservoirs with adjacent rock with a higher concentration of uranium, for example granitic minerals. It should be mentioned that α-recoil mechanism is efficient when dissolution rates are low and the residence times of adjacent water in contact with the rock are very long. Such conditions do not exist in the case of Poddebice thermal groundwater, which has a temperature of about 60 °C, and a sandy soil environment with very low uranium content. The profile of the uranium activity concentration in the Poddebice bore-hole is shown in Fig. 5.
As is evident from Fig. 5, uranium surface soil activity is equal to 17 Bq/kg, whereas for the deeper sandy geological formation, which surround the thermal groundwater reservoir, this activity concentration decreases to very low values of ≈11 Bq/kg.
In this case, the dissolution rate of both uranium isotopes is higher and the input of 234U recoil directly to the solution is negligible. However, because of very low uranium concentration in the surrounding soil, leachable uranium radionuclides in the time scale of these processes (the dozen thousands of years) are exhausted and there is no renewal of material (uranium) exposed to water weathering. Then, such water would be expected to evolve to have values of 234U/238U ratio below unity even up to 0.3 as presented in models by Anderson et al. [24] or Rihs et al. [25].
This data clearly show lack of any water transport from the surface water to thermal groundwater reservoir. However, such transfer from the Ner river to the underground municipal water is possible.
Uranium activity concentration in thermal groundwater samples from Central Poland
Lower values of 234U/238U activity ratio were also observed for the geothermal waters in other places in Central Poland. The results are presented in Table 3.
As shown in Table 3, the activity concentration of 234U and 238U in water samples varied in a wide range, from <0.013 to 16.84 mBq/dm3 and from <0.013 to 45.47 mBq/dm3, respectively. The highest uranium activity concentrations were measured in thermal groundwater from Mszczonow and Cieplice, whereas the lowest uranium activities were found in thermal ground water from Uniejow and Poddebice.
The values of 234U/238U activity ratio below 1 confirm basically the same hydro geochemical conditions for all these thermal groundwater reservoirs in Central Poland. Similarly, lower values of uranium activity ratio for most geothermal waters are reported by Osmond and Cowart [4].
Conclusions
234U and 238U activity concentrations were determined in the water samples (river water, deep well water and thermal groundwater) collected from Central Poland. Uranium activity concentrations in the analyzed waters varied in a wide range. On the basis of the measured values, the uranium activity ratio (234U/238U) was calculated. In deep well water and river water, uranium activity ratio (234U/238U) was higher than 1 (from 1.06 to 1.20), while in thermal groundwater it was lower than 1 (from 0.75 to 0.95). A comparison of the average uranium isotopic ratio in the well water from Poddebice (1.06) and river water from the Ner (1.09) show possible infiltration of surface water to this reservoir.
The lower 234U/238U ratios in the geothermal water are caused by the fact that the majority of both uranium isotopes come from the uranium present in low concentrations in the adjacent rocks and its relatively quick dissolving at higher temperatures leads to its exhausting from the surface layer of rocks contacting with water. Therefore, the input of the recoil 234U atoms is negligible. The remarkably lower 234U/238U data for thermal groundwater in the Poddebice region in comparison with data for surface and well water in this region, clearly show lack of any water transport from the surface water to this reservoir.
References
Camacho A, Devesa R, Valles I, Serrano I, Soler J, Blazquez S, Ortega X, Matia L (2010) Distribution of uranium isotopes in surface water of the Llobregat river basin (Northeast Spain). J Environ Radioact 101:1048–1054
Skwarzec B, Boryło A, Strumińska D (2002) 234U and 238U isotopes in water and sediments of the southern Baltic. J Environ Radioact 61:345–363
Plater AJ, Ivanovich M, Dugdale RE (1992) Uranium series disequilibrium in river sediments and waters: the significance of anomalous activity ratios. Appl Geochem 7:101–110
Osmond JK, Cowart JB (1992) Groundwater. In: Ivanovich M, Harmon RS (eds) Uranium-series disequilibrium. Applications to earth, marine and environmental sciences, 2nd edn. Oxford Science Publications, Oxford
Fleischer RL (1980) Isotopic disequilibrium of uranium: alpha-recoil damage and preferential solution effects. Science 207:979–981
Pietrzak-Flis Z, Kamińska I, Chrzanowski E (2004) Uranium isotopes in waters and bottom sediments of rivers and lakes in Poland. Nukleonika 49(2):69–76
Bellis D, Ma R, Bramall N, McLeod CW (2001) Airborne emission of enriched uranium at Tokai-mura, Japan. Sci Tot Environ 114:283–286
Mas JL, Ma R, McLeod C, González-Labajo J, Cox A, Watson P (2006) Determination of 234U/238U isotope ratios in environmental waters by quadrupole ICP-MS after U stripping from alpha-spectrometry counting sources. Anal Bioanal Chem 386:152–160
Zoriy MV, Halicz L, Ketterer ME, Pickhartd C, Ostapczuk P, Becker JS (2004) Reduction of UH+ formation for 236U/238U isotope ratio measurements at ultratrace level in double focusing sector field ICP-MS using D2O as solvent. J Anal Atom Spectrom 19:362–367
UNESCAR (2000) United Nations Committee on the Effects of Atomic Radiation. Sources, Effects, and Risks of Ionizing Radiation. United Nations, New York
Hossain MMM, Ohde S (2002) Uranium in pore water from coastal sediments determined by chemical activation analysis. J Radioanal Nucl Chem 251(2):333–336
Vesterbacka P (2005) 238U-series radionuclides in Finnish groundwater-based drinking water and effective doses. STUK, Helsinki
Rathore DPS (2008) Advanced in technologies for the measurement of uranium in divorce matrices. Talanta 77:9–20
Hou X, Roos P (2008) Critical comparison of radiometric and mass spectrometric method for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal Chim Acta 608:105–139
Becker JS (2005) Inductively coupled plasma mass spectrometry (ICP-MS) and laser ablation ICP-MS for isotope analysis of long-lived radionuclides. Int J Mass Spectrom 242:183–195
Grabowski P, Długosz M, Szajerski P, Bem H (2010) A comparison of selected natural radionuclide concentrations in the thermal groundwater of Mszczonów and Cieplice with deep well water from Łódź city, Poland. Nukleonika 55(2):181–185
Skwarzec B, Strumińska DI, Boryło A (2003) Radionuclides of 210Po, 234U and 238U in drinking bottled mineral water in Poland. J Radioanal Nucl Chem 256(2):361–364
Eikenberg J, Tricca A, Vezzu G, Bajo S, Ruethi M, Surbeck (2001) Determination of 228Ra, 226Ra and 224Ra in natural water via adsorption on MnO2-coated discs. J Environ Radioact 54:109–131
Bem H, Olszewski M, Kaczmarek A (2004) Concentration of selected natural radionuclides in the thermal groundwater of Uniejow. Nukleonika 49(1):1–5
Jia G, Torri G, Ocone R, Di Lullo A, De Angelis A, Boschetto R (2008) Determination of thorium isotopes in mineral and environmental water and soil samples by a-spectrometry and the fate of thorium in water. Appl Radiat Isot 66:1478–1487
Skwarzec B, Kabat K, Astel A (2009) Seasonal and spatial variability of 210Po, 238U and 239+240Pu levels in the river catchment area assessed by application of neural-network based classification. J Environ Radioact 100:167–175
Kigoshi K (1971) Alpha-recoil thorium-234: dissolution into water and the uranium-234/uranium-238 disequilibrium in nature. Science 173:47–48
Fleischer RL, Raabe OG (1978) Recoiling alpha-emitting nuclei. Mechanisms for uranium series disequilibrium. Geochem Cosmochim Acta 42:973–978
Andersen MB, Erel Y, Bourdon B (2009) Experimental evidence for 234U–238U fractionation during granite weathering with implications for 234U/238U in natural waters. Geochem Cosmochim Acta 73:4124–4141
Rihs S, Condomines M, Poidevin JL (2000) Long-term behavior of continental hydrothermal systems: U-series study of hydrothermal carbonates from the French Massif Central (Allier Valley). Geochem Cosmochim Acta 64(18):3189–3199
Acknowledgment
We gratefully acknowledge the financial support by the Protection of the Environment and Water Management Fund in Łódź and Polish Ministry of Higher Education (KBN) grant 1341/B/H03/2011/40.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Grabowski, P., Bem, H. Uranium isotopes as a tracer of groundwater transport studies. J Radioanal Nucl Chem 292, 1043–1048 (2012). https://doi.org/10.1007/s10967-011-1558-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1558-0