Abstract
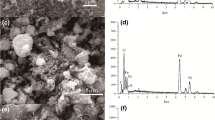
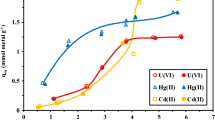
Both metal hydroxides and chitosan have been recognized as effective scavengers for nuclide ions. However, the aggregation of metal hydroxides and the weak mechanical strength of chitosan beads greatly restricts their application. Herein, the in-situ formed Ni(OH)2 nanoparticles was immobilized in chitosan matrix to obtain a novel hybrid sorbent (CSNi) for U(VI) adsorption. The CSNi showed high affinity for U(VI) sorption, with the inner-sphere complexation as the main mechanism. The maximum mono-layer adsorption capacity for U(VI) at 298 K reached 164.2 mg/g. The U(VI) sorption was endothermic and spontaneous, and kinetically followed the pseudo-second-order model. These findings highlight the possibility of using CSNi for efficient removal of U(VI) from wastewater.







Similar content being viewed by others
References
Tan L, Zhang X, Liu Q, Jing X, Liu J, Song D, Wang J (2015) Synthesis of Fe3O4@TiO2 core–shell magnetic composites for highly efficient sorption of uranium (VI). Colloids Surf A 469:279–286
Wang X, Fan Q, Yu S, Chen Z, Ai Y, Sun Y, Wang X (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455
Zhang C, Dodge CJ, Malhotra SV, Francis AJ (2013) Bioreduction and precipitation of uranium in ionic liquid aqueous solution by Clostridium sp. Bioresour Technol 136:752–756
Quinn JE, Wilkins D, Soldenhoff KH (2013) Solvent extraction of uranium from saline leach liquors using DEHPA/Alamine 336 mixed reagent. Hydrometallurgy 134:74–79
Pulhani VA, Dafaut S, Hegde AG (2011) Separation of uranium from iron in ground water samples using ion exchange resins. J Radioanal Nucl Chem 294:299–302
Kedari CS, Pandit SS, Gandhi PM (2013) Separation by competitive transport of uranium(VI) and thorium(IV) nitrates across supported renewable liquid membrane containing trioctylphosphine oxide as metal carrier. J Membr Sci 430:188–195
Ji G, Zhu G, Wang X, Wei Y, Yuan J, Gao C (2017) Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Sep Purif Technol 174:455–465
Sun Y, Zhang R, Ding C, Wang X, Cheng W, Chen C, Wang X (2016) Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: a combined batch, EXAFS and modeling techniques. Geochim Cosmochim Acta 180:51–65
Mallakpour S, Madani M (2016) Functionalized-MnO2/chitosan nanocomposites: a promising adsorbent for the removal of lead ions. Carbohydr Polym 147:53–59
Vincent C, Hertz A, Vincent T, Barré Y, Guibal E (2014) Immobilization of inorganic ion-exchanger into biopolymer foams–Application to cesium sorption. Chem Eng J 236:202–211
Elabd AA, Zidan WI, Abo-Aly MM, Bakier E, Attia MS (2014) Uranyl ions adsorption by novel metal hydroxides loaded Amberlite IR120. J Environ Radioact 134:99–108
Pan B, Qiu H, Nie G, Xiao L, Lv L, Zheng S (2010) Highly efficient removal of heavy metals by polymer-supported nanosized hydrated Fe(III) oxides: behavior and XPS study. Water Res 44:815–824
Shao D, Ren X, Wen J, Hu S, Xiong J, Jiang T, Wang X (2016) Immobilization of uranium by biomaterial stabilized FeS nanoparticles: effects of stabilizer and enrichment mechanism. J Hazard Mater 302:1–9
Roques J, Veilly E, Simoni E (2009) Periodic density functional theory investigation of the uranyl ion sorption on three mineral surfaces: a comparative study. Int J Mol Sci 10:2633–2661
Dudarko OA, Gunathilake C, Wickramaratne NP, Sliesarenko VV, Zub YL, Górka J, Dai S, Jaroniec M (2015) Synthesis of mesoporous silica-tethered phosphonic acid sorbents for uranium species from aqueous solutions. Colloids Surf A Physicochem Eng 482:1–8
Galhoum AA, Atia AA, Mahfouz MG, Abdel-Rehem ST, Gomaa NA, Vincent T, Guibal E (2015) Dy(III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amine acids. J Mater Sci 50:2832–2848
Mahfouz MG, Galhoum AA, Gomaa NA, Abdel-Rehem SS, Atia AA, Vincent T, Guibal E (2015) Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: equilibrium and kinetic studies. Chem Eng J 262:198–209
Kyzas GZ, Kostoglou M, Lazaridis NK, Bikiaris DN (2013) N-(2-Carboxybenzyl) grafted chitosan as adsorptive agent for simultaneous removal of positively and negatively charged toxic metal ions. J Hazard Mater 244:29–38
Anirudhan TS, Radhakrishnan PG (2009) Improved performance of a biomaterial-based cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100:250–257
Sorlier P, Viton C, Domard A (2002) Relation between solution properties and degree of acetylation of chitosan: role of aging. Biomacromol 3:1336–1342
Zhao Y, Wang X, Li J, Wang X (2015) Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polym Chem 6:5376–5384
Xu J, Chen M, Zhang C, Yi Z (2013) Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298:1375–1383
Rahmati A, Ghaemi A, Samadfam M (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy 39:42–48
Stopa LC, Yamaura M (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5:283–289
Hosoba M, Oshita K, Katarina RK, Takayanagi T, Oshima M, Motomizu S (2009) Synthesis of novel chitosan resin possessing histidine moiety and its application to the determination of trace silver by ICP-AES coupled with triplet automated-pretreatment system. Anal Chim Acta 639:51–56
Wang J, Peng R, Yang J, Liu Y, Hu X (2011) Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr Polym 4:1169–1175
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198:412–419
Sureshkumar MK, Das D, Mallia MB, Gupta PC (2010) Adsorption of uranium from aqueous solution using chitosan- tripolyphosphate (CTPP) beads. J Hazard Mater 184:65–72
Akkaya R, Ulusoy U (2008) Adsorptive features of chitosan entrapped in polyacrylamide hydrogel for Pb2+, UO2 2+, and Th4+. J Hazard Mater 151:380–388
Sabarudin A, Oshima M, Takayanagi T, Hakim L, Oshita K, Gao YH, Motomizu S (2007) Functionalization of chitosan with 3,4-dihydroxybenzoic acid for the adsorption/collection of uranium in water samples and its determination by inductively coupled plasma-mass spectrometry. Anal Chim Acta 581:214–220
Acknowledgements
This work was financially supported by The National Natural Science Fund (21366001; 21667001), The International Scientific and Technological Cooperation Projects (2015DFR61020), The Key Research and Development Program and The Natural Science Fund Program of Jiangxi Province (20161BBF60059; S2017ZRMSB0473).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, L., Li, Z., Zeng, K. et al. Immobilization of in-situ formed Ni(OH)2 nanoparticles in chitosan beads for efficient removal of U(VI) from aqueous solutions. J Radioanal Nucl Chem 314, 467–476 (2017). https://doi.org/10.1007/s10967-017-5407-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5407-7