Abstract
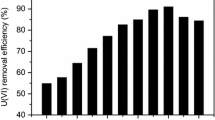
This study had been devoted to investigate the adsorption capacity and adsorption mechanism of Elodea nuttallii. Experimental investigation related to the adsorption behaviors of E. nuttallii toward uranium, regeneration and U(VI) adsorption capacity. The results showed that with the initial U(VI) concentration increased, the maximum adsorption capacity was reached at 3.92 mg/g, regeneration adsorption rate reached 55%, adsorption of U(VI) progress could be described by the Langmuir model and pseudo-second-order kinetics. FT-IR analysis indicated that multiple functional groups involve together in the process of adsorbing U(VI). Therefore, E. nuttallii is a potential absorbent for U(VI) removal from aqueous solution.









Similar content being viewed by others
References
Yang Y, Meng J (2017) Environment pollution state and improvement measures in rural areas of heilongjiang, China. Nat Environ Pollut Technol 16(4):1087–1093
Li F, Li X, Cui P (2018) Detoxification of U(VI) by Paecilomyces catenlannulatus investigated by batch, XANES and EXAFS techniques. J Environ Radioactiv 189:24–30
Minas F, Chandravanshi BS, Leta S (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem Int 3(4):291–305
Sar SK, Diwan V, Biswas S, Singh S, Sahu M, Jindal MK, Arora A (2018) Study of uranium level in groundwater of Balod district of Chhattisgarh state, India and assessment of health risk. Hum Ecol Risk Assess 24:691–698
Hamutoko JT, Mapani BS, Ellmies R, Bittner A, Kuells C (2014) A fingerprinting method for the identification of uranium sources in alluvial aquifers: an example from the Khan and Swakop Rivers, Namibia. Phys Chem Earth 72–75:34–42
Nada FT, Laith AN, Enas MY (2014) Uranium concentration and its associated health hazards in drinking water of Nineveh Province (Iraq). World Appl Sci J 31(11):1938–1944
Fan F-L, Qin Z, Bai J, Rong WD, Fan FY, Tian W, Wu XL, Wang Y, Zhao L (2012) Rapid removal of uranium from aqueous solutions using magnetic Fe3O4@SiO2 composite particles. J Environ Radioactiv 106:40–46
Xie Y, Ren L, Zhu X, Gou X, Chen SY (2018) Physical and chemical treatments for removal of perchlorate from water—a review. Process Saf Environ 116:180–198
Mojiri A, Aziz HA, Zaman NQ, Aziz SQ, Zahed MA (2016) Metals removal from municipal landfill leachate and wastewater using adsorbents combined with biological method. Desalin Water Treat 57:2819–2833
Charles W, Ho G (2017) Biological methods of odor removal in solid waste treatment facilities. Curr Dev Biotechnol Bioeng. https://doi.org/10.1016/B978-0-444-63664-5.00015-0
Sharma P, Pandey S (2014) Status of phytoremediation in world scenario. Int J Environ Biorem Biodegrad 2(4):178–191
Li Y, Zhao J, Guo J, Liu MJ, Xu QL, Li H, Li YF, Zheng L, Zhang ZY, Gao YX (2017) Influence of sulfur on the accumulation of mercury in rice plant (Oryza sativa L.) growing in mercury contaminated soils. Chemosphere 182:293–300
Sharma S, Singh B, Thulasidas SK, Kulkarni MJ, Natarajan V, Manchanda VK (2016) Evaluation of terrestrial plants extracts for uranium sorption and characterization of potent phytoconstituents. Int J Phytoremediat 18:10–15
Chen BD, Zhu Y-G, Smith FA (2006) Effects of arbuscular mycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pteris vittata L.) from a uranium mining-impacted soil. Chemosphere 62:1464–1473
Mkandawire M, Taubert B, Dudel EG (2004) Capacity of Lemna gibba L. (Duckweed) for uranium and arsenic phytoremediation in mine tailing waters. Int J Phytoremediat 6:347–362
Schaumburg J, Schranz C, Foerster J, Gutowski A, Hofmann G, Meilinger P, Schneider S, Schmedtje U (2004) Ecological classification of macrophytes and phytobenthos for rivers in Germany according to the water framework directive. Limnologica 34:283–301
Jha VN, Tripathi RM, Sethy NK, Sahoo SK (2016) Uptake of uranium by aquatic plants growing in fresh water ecosystem around uranium mill tailings pond at Jaduguda, India. Sci Total Environ 539:175–184
Singh D, Gupta R, Tiwari A (2011) Phytoremediation of lead from wastewater using aquatic plants. Int J Biomed Res 2:411–421
Abu Bakar AF, Yusoff I, Fatt NT, Othman F, Ashraf MA (2013) Arsenic, zinc, and aluminium removal from gold mine wastewater effluents and accumulation by submerged aquatic plants (Cabomba piauhyensis, Egeria densa, and Hydrilla verticillata). Biomed Res Int 2013:1–7
Kunii H (1984) Seasonal growth and profile structure development of Elodea nuttallii (Planch.) St, John in pond Ojaga-ike, Japan. Aquat Bot 18:239–247
Angelstein S, Schubert H (2008) Elodea nuttallii: uptake, translocation and release of phosphorus. Aquat Biol 3:209–216
Yi ZJ, Yao J, Zhu MJ, Chen HL, Wang F, Yuan ZM, Liu X (2016) Batch study of uranium biosorption by Elodea canadensis biomass. J Radioanal Nucl Chem 310(2):1–9
Saha P, Shinde O, Sarkar S (2017) Phytoremediation of industrial mines wastewater using water hyacinth. Int J Phytoremediat 19:87–96
Imtiaz M, Ashraf M, Rizwan MS, Nawaz MA, Rizwan M, Mehmood S, Yousaf B, Yuan Y, Ditta A, Mumtaz MA, Ali M, Mahmood S, Tu SX (2018) Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: effects on cell death, ROS and antioxidative systems. Ecotox Environ Safe 158:139–144
Fathi RA, Godbold DL, Al-Salih HS, Jones D (2014) Potential of Phytoremediation to clean up uranium-contaminated soil with Acacia species. Res J Environ Earth Sci 4:82–91
Li GY, Hu N, Ding DX, Zheng JF, Liu YL, Wang YD, Nie XQ (2011) Screening of plant species for phytoremediation of uranium, thorium, barium, nickel, strontium and lead contaminated soils from a uranium mill tailings repository in South China. Bull Environ Contam Toxicol 86:646–652
Mkandawire M, Taubert B, Dudel EG (2004) Capacity of Lemna gibba L. (Duckweed) for uranium and arsenic phytoremediation in mine tailing waters. Int J Phytorem 6:347–362
Danh LT, Truong P, Mammucari R, Foster N (2014) A critical review of the arsenic uptake mechanisms and phytoremediation potential of Pteris vittata. Int J Phytorem 16:429–453
Department of Agronomy, J.N.K.V.V., Jabalpur (M.P.), Rajput A (2017) Physiological parameters leaf area index, crop growth rate, relative growth rate and net assimilation rate of different varieties of rice grown under different planting geometries and depths in SRI. Int J Pure Appl Biosci 5:362–367
Marwani HM, Albishri HM, Jalal TA, Soliman EM (2017) Study of isotherm and kinetic models of lanthanum adsorption on activated carbon loaded with recently synthesized Schiff’s base. Arab J Chem 10:S1032–S1040
Geider R, Graziano L, Michaelmchay R (1998) Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur J Phycol 33(4):315–322
Zhou HP, Zhao JF, Yang YQ, Chen CX, Liu YF, Jin XH, Chen LM, Li XY, Deng XW, Schumaker KS, Guo Y (2012) Ubiquitin-specific protease16 modulates salt tolerance in arabidopsis by regulating Na+/H+ antiport activity and serine hydroxymethyltransferase stability. Plant Cell 24(12):5106–5122
Feiler U, Hoss S, Ahlf W, Gilberg D, Hammers-Wirtz M, Hollert H, Meller M, Neumann-Hensel H, Ottermanns R, Seiler TB, Spira D, Heininger P (2013) Sediment contact tests as a tool for the assessment of sediment quality in German waters. Environ Toxicol Chem 32(1):144–155
Wołowicz A, Hubicki Z (2012) Applicability of new acrylic, weakly basic anion exchanger purolite A-830 of very high capacity in removal of palladium(II) chloro-complexes. Ind Eng Chem Res 51:7223–7230
Singh J, Sharma M, Basu S (2018) Heavy metal ions adsorption and photodegradation of remazol black XP by iron oxide/silica monoliths: kinetic and equilibrium modelling. Adv Powder Technol 29:2268–2279
Bellam R, Jaganyi D, Mambanda A, Robinson R (2018) Role of a 2,3-bis(pyridyl)pyrazinyl chelate bridging ligand in the reactivity of Ru(ii)–Pt(ii) dinuclear complexes on the substitution of chlorides by thiourea nucleophiles—a kinetic study. New J Chem 42:12557–12569
Kučić D (2018) Batch adsorption of Cr(VI) ions on zeolite and agroindustrial waste. Chem Biochem Eng Q 31:497–507
Toyoshima Y (2017) Origin of pseudo second order reaction in a-Si: H growth. e-J Surf Sci Nanotechnol 15:93–95
Huang R, Liu Q, Huo J, Yang B (2017) Adsorption of methyl orange onto protonated cross-linked chitosan. Arab J Chem 10:24–32
Yuan GY, Tian Y, Liu J, Tu H, Liao JL, Yang JJ, Yang YY, Wang DQ, Liu N (2017) Schiff base anchored on metal-organic framework for Co (II) removal from aqueous solution. Chem Eng J 326:691–699
Ngee LH, Kassim A, Ming HN, Abdullah DK, Abdullah AH, Yarmo MA, Kian YS (2008) Phase behavioural study of palm-based lauryl alcohol ethoxylates. Pertanika J Sci Technol 16(2):141–156
Hirmizi NHM, Bakar MA, Tan WL, Ismail J, See CH (2012) Electrical and thermal behavior of copper-epoxy nanocomposites prepared via aqueous to organic phase transfer technique. J Nanomater 11:1–11
Schmeide K, Sachs S, Bubner M, Reich T, Heise KH, Bernhard G (2003) Interaction of uranium(VI) with various modified and unmodified natural and synthetic humic substances studied by EXAFS and FTIR spectroscopy. Inorg Chim Acta 351(1):133–140
Ganesh S, Prakash S, Jayakumar R (2003) Spectroscopic investigation on gel-forming β-sheet assemblage of peptide derivatives. Biopolymers 70(3):346–354
Patel NB, Patel SD, Patel AL, Patel JC, Patel JN (2011) Synthesis and antimicrobial studies of Schiff bases of fluoroquinolone. India J Chem 50(11):1645–1657
Cabrera-Lara LI (2017) Reaction parameters for controlled sonosynthesis of gold nanoparticles. J Mex Chem Soc 59:119–129
Frost RL, Bahfenne S, Čejka J, Sejkora J, Plásil J, Palmer SJ, Keeffe EC, Němec I (2011) Dussertite BaFe3 3+(AsO4)2(OH)5-a Raman spectroscopic study of a hydroxy-arsenate mineral. J Raman Spectrosc 42:56–61
Černe M, Smodiš B, Štrok M (2011) Uptake of radionuclides by a common reed (Phragmites australis (Cav.) Trin. ex Steud.) grown in the vicinity of the former uranium mine at Žirovski vrh. Nucl Eng Des 241:1282–1286
Favas PJC, Pratas J (2013) Uptake of uranium by native aquatic plants: potential for bioindication and phytoremediation. E3S Web Conf 1:13007 p1–p3
Acknowledgements
This work was supported by the Country National Defense Fundamental Researh Program (Grant 16ZG6101). The Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology have offered aid, the authors present our sincere thanks here.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest to this work.
Rights and permissions
About this article
Cite this article
Yang, H., Luo, X., Ding, H. et al. Adsorption of U(VI) by Elodea nuttallii: equilibrium, kinetic and mechanism analysis. J Radioanal Nucl Chem 319, 227–235 (2019). https://doi.org/10.1007/s10967-018-6346-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6346-7