Abstract
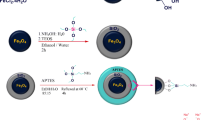
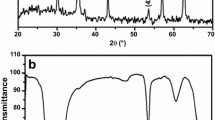
FeS@Fe3O4 magnetic nanoparticles were prepared by ultrasonic-assisted method and characterized by TEM, FTIR, XRD, SEM, EDS, BET and VSM. The factors affecting the adsorption properties of uranyl ions by FeS@Fe3O4 were studied. Results show that the FeS@Fe3O4 nanoparticles have core–shell structure and superparamagnetism. Under the optimized conditions, the maximum adsorption capacity can reach 229.03 mg/g. The optimum adsorption conditions were as follows: pH = 6, temperature 80 °C, C0 = 35 mg/L, contact time 2.5 h, adsorbent dosage 10 mg. Adsorption kinetics and thermodynamic studies show that the adsorption process accords with the Freundlich isotherm adsorption model and the pseudo-second-order kinetic model.
















Similar content being viewed by others
References
Feng ML, Sarma D, Qi X, Du KZ, Huang XY, Kanatzidis MG (2016) Efficient removal and recovery of uranium by a layered organic-inorganic hybrid thiostannate. J Am Chem Soc 138(38):12578–12585
Tavengwa NT, Cukrowska E, Chimuka L (2015) Sequestration of U(VI) from aqueous solutions using precipitate ion imprinted polymers endowed with oleic acid functionalized magnetite. J Radioanal Nucl Chem 304(2):933–943
Gong Y, Tang J, Zhao D (2016) Application of iron sulfide particles for groundwater and soil remediation: a review. Water Res 89:309–320
Liu R, Yang Z, He Z, Wu L, Hu C, Wu W, Qu J (2016) Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): inhibitive effects of S(0)-enriched surfaces. Chem Eng J 304:986–992
Suzuki T, Kawasaki T, Takao K, Harada M, Nogami M, Ikeda Y (2012) A study on selective precipitation ability of cyclic urea to U(VI) for developing reprocessing system based on precipitation method. J Nucl Sci Technol 49(10):1010–1017
Tran TK, Leu HJ, Chiu KF, Lin CY (2017) Electrochemical treatment of heavy metal-containing wastewater with the removal of COD and heavy metal ions. J Chin Chem Soc 64(5):493–502
Chen L, Bai Z, Zhu L, Zhang L, Cai Y, Li Y, Liu W, Wang Y, Chen L, Diwu J (2017) Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework. ACS Appl Mater Interfaces 9(38):32446–32451
Endrizzi F, Leggett C, Rao L (2016) Scientific basis for efficient extraction of uranium from seawater, I: understanding the chemical speciation of uranium under seawater conditions. Ind Eng Chem Res 55(15):4249–4256
Wang LL, Luo F, Dang LL, Li JQ, Wu XL, Liu SJ, Luo MB (2015) Correction: ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide- and carboxyl-functionalized metal–organic framework. J Mater Chem A 3(34):17880
Zhe X, Jiangtao HU, Wang MH, Zhang WL, Shineng LI, Gao QH, Guozhong WU (2013) Properties and evaluation of amidoxime-based UHMWPE fibrous adsorbent for extraction of uranium from seawater. Sci China Chem 56(11):1504–1509
Hoyer M, Zabelt D, Steudtner R, Brendler V, Haseneder R, Repke JU (2014) Influence of speciation during membrane treatment of uranium contaminated water. Sep Purif Technol 132:413–421
Torkabad MG, Keshtkar AR, Safdari SJ (2017) Comparison of polyethersulfone and polyamide nanofiltration membranes for uranium removal from aqueous solution. Prog Nucl Energy 94:93–100
Ma H, Hsiao BS, Chu B (2013) Ultrafine cellulose nanofibers as efficient adsorbents for removal of UO2 2+ in water. ACS Macro Lett 1(1):213–216
Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B (2011) Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J 171(3):1185–1193
Kaynar ÜH, Ayvacıklı M, Kaynar SÇ, Hiçsönmez Ü (2014) Removal of uranium(VI) from aqueous solutions using nanoporous ZnO prepared with microwave-assisted combustion synthesis. J Radioanal Nucl Chem 299(3):1469–1477
Jing L, Lei Z, Dong F, Hudson-Edwards KA (2016) Enhancing As(V) adsorption and passivation using biologically formed nano-sized FeS coatings on limestone: implications for acid mine drainage treatment and neutralization. Chemosphere 168:529–538
Liu X, Ai L, Jiang J (2015) Interconnected porous hollow CuS microspheres derived from metal-organic frameworks for efficient adsorption and electrochemical biosensing. Powder Technol 283:539–548
Qu Z, Yan L, Li L, Xu J, Liu M, Li Z, Yan N (2014) Ultraeffective ZnS nanocrystals sorbent for mercury(II) removal based on size-dependent cation exchange. ACS Appl Mater Interfaces 6(20):18026–18032
Wolthers M, Charlet L, Linde PRvD, Rickard D, Weijden CHvD (2005) Surface chemistry of disordered mackinawite (FeS). Geochim Cosmochim Acta 69(14):3469–3481
Watson JHP, Ellwood DC, Deng Q, Mikhalovsky S, Hayter CE, Evans J (1995) Heavy metal adsorption on bacterially produced FeS. Miner Eng 8(10):1097–1108
Fang L, Li L, Qu Z, Xu H, Xu J, Yan N (2018) A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J Hazard Mater 342:617–624
Sun Y, Liu Y, Lou Z, Yang K, Lv D, Zhou J, Xu X (2018) Enhanced performance for Hg(II) removal using biomaterial (CMC/gelatin/starch) stabilized FeS nanoparticles: stabilization effects and removal mechanism. Chem Eng J 344:616–624
Dzade NY, Roldan A, Leeuw NHD (2017) Structures and properties of As(OH)3 adsorption complexes on hydrated mackinawite (FeS) surfaces: a DFT-D2 study. Environ Sci Technol 51(6):3461–3470
Shao D, Ren X, Wen J, Hu S, Xiong J, Jiang T, Wang X, Wang X (2016) Immobilization of uranium by biomaterial stabilized FeS nanoparticles: effects of stabilizer and enrichment mechanism. J Hazard Mater 302:1–9
Sun Y, Lou Z, Yu J, Zhou X, Lv D, Zhou J, Baig SA, Xu X (2017) Immobilization of mercury (II) from aqueous solution using Al2O3-supported nanoscale FeS. Chem Eng J 323:483–491
Chen L, Zhao D, Chen S, Wang X, Chen C (2016) One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal. J Colloid Interface Sci 472:99–107
Yan LG, Yang K, Shan RR, Yan T, Wei J, Yu SJ, Yu HQ, Du B (2015) Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J Colloid Interface Sci 448:508–516
Zhang D, Gao G, Ma W, Zhu J, Qiu G, Liu X (2013) Facile synthesis and properties of bifunctional magnetic-optical Fe3O4@ZnS nanocomposites with core-shell structure. Appl Mech Mater 320:92–98
Zhao D, Zhang Q, Xuan H, Chen Y, Zhang K, Feng S, Alsaedi A, Hayat T, Chen C (2017) EDTA functionalized Fe3O4/graphene oxide for efficient removal of U(VI) from aqueous solutions. J Colloid Interface Sci 506:300–307
Tavengwa NT, Cukrowska E, Chimuka L (2016) Modeling of adsorption isotherms and kinetics of uranium sorption by magnetic ion imprinted polymers. Toxicol Environ Chem Rev 98(1):1–12
Kera NH, Bhaumik M, Pillay K, Ray SS, Maity A (2017) Selective removal of toxic Cr(VI) from aqueous solution by adsorption combined with reduction at a magnetic nanocomposite surface. J Colloid Interface Sci 503:214–228
Yu Y, Yu L, Shih K, Chen JP (2018) Yttrium-doped iron oxide magnetic adsorbent for enhancement in arsenic removal and ease in separation after applications. J Colloid Interface Sci 521:252–260
Zhang S, Zhang Y, Liu J, Xu Q, Xiao H, Wang X, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226(24):30–38
Iranmanesh P, Saeednia S, Mehran M, Dafeh SR (2016) Modified structural and magnetic properties of nanocrystalline MnFe2O4 by pH in capping agent free co-precipitation method. J Magn Magn Mater 425:31–36
Xu Y, Ke G, Yin J, Lei W, Yang P (2019) Synthesis of thiol-functionalized hydrotalcite and its application for adsorption of uranium (VI). J Radioanal Nucl Chem 319(3):791–803
Wang CF, Liu ZR, Xue GR, Lei Y, Wang Y, Zhou LM (2016) Adsorptive properties of sunflower seed shells for UO2 2+ in aqueous solution. J Nucl Radiochem 38(2):107–115
Gholizadeh A, Jafari E (2017) Effects of sintering atmosphere and temperature on structural and magnetic properties of Ni-Cu-Zn ferrite nano-particles: magnetic enhancement by a reducing atmosphere. J Magn Magn Mater 422:328–336
Ma YX, Xing D, Shao WJ, Du XY, La PQ (2017) Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg(II) in aqueous solution. J Colloid Interface Sci 505:352–363
Li SL, Zhou YP, Liu JJ (2016) Physical chemistry (2009), 5th edn. Higher Education Press, Beijing, pp 594–595
Gao Y, Yuan Y, Ma D, Li L, Li Y, Xu W, Tao W (2014) Removal of aqueous uranyl ions by magnetic functionalized carboxymethylcellulose and adsorption property investigation. J Nucl Mater 453(1–3):82–90
Dolatyari L, Yaftian MR, Rostamnia S (2016) Removal of uranium(VI) ions from aqueous solutions using Schiff base functionalized SBA-15 mesoporous silica materials. J Environ Manage 169:8–17
Lu BQ, Li M, Zhang XW, Huang CM, Wu XY, Fang Q (2018) Immobilization of uranium into magnetite from aqueous solution by electrodepositing approach. J Hazard Mater 343:255–265
Sepehrian H, Asadi Z (2012) Studies on the recovery of uranium from nuclear industrial effluent using nanoporous silica adsorbent. Int J Environ Sci Technol 9(4):629–636
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366(1):165–172
Qian J, Zhang S, Zhou Y, Dong P, Hua D (2014) Synthesis of surface ion-imprinted magnetic microspheres by locating polymerization for rapid and selective separation of uranium(VI). RSC Adv 5(6):4153–4161
Khani R, Sobhani S, Beyki MH (2016) Highly selective and efficient removal of lead with magnetic nano-adsorbent: multivariate optimization, isotherm and thermodynamic studies. J Colloid Interface Sci 466:198–205
Donia AM, Atia AA, El-Boraey H, Mabrouk DH (2006) Uptake studies of copper(II) on glycidyl methacrylate chelating resin containing Fe2O3 particles. Sep Purif Technol 49(1):64–70
Zhang X, Wang J, Li R, Dai Q, Gao R, Liu Q, Zhang M (2013) Preparation of Fe3O4@C@layered double hydroxide composite for magnetic separation of uranium. Ind Eng Chem Res 52(30):10152–10159
Acknowledgements
This study was financially supported by Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol Fiber Material, Huaihua University (HGY201805) and the Natural Science Foundation of Hunan Province (2017JJ2231).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Yang, P., Li, Q. et al. Preparation of FeS@Fe3O4 core–shell magnetic nanoparticles and their application in uranyl ions removal from aqueous solution. J Radioanal Nucl Chem 321, 499–510 (2019). https://doi.org/10.1007/s10967-019-06626-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06626-2