Abstract
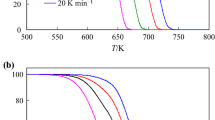
The thermooxidative degradation kinetics of poly(tetrafluoroethene) (PTFE) in air flow has been studied at different heating rates (6, 10, 12 and 15 K min−1) by non-isothermal differential thermal analysis (DTA). Six calculation procedures based on single TG curves and iso-conversional method, as well as 27 mechanism functions were used. The comparison of the results obtained with these calculation procedures showed that they strongly depend on the selection of proper mechanism function for the process. Therefore, it is very important to determine the most probable mechanism function. In this respect the iso-conversional calculation procedure turned out to be more appropriate. In the present work, the values of apparent activation energy E, pre-exponential factor A in Arrhenius equation, as well as the changes of entropy ΔS ≠, enthalpy ΔH ≠ and free Gibbs energy ΔG ≠ for the formation of the activated complex from the reagent are calculated. All calculations were performed using programs compiled by ourselves.









Similar content being viewed by others
References
Conesa JA, Font R. Polytetrafluoroethylene decomposition in air and nitrogen. Polym Eng Sci. 2001;41:2137–47.
Simon CM, Kaminsky W. Chemical recycling of polytetrafluoroethylene by pyrolysis. Polym Degrad Stab. 1998;62:1–7.
Baker BB, Kasprzak DJ. Thermal degradation of commercial fluoropolymers in air. Polym Degrad Stab. 1993; 42:181–8.
Ksiazczak A, Boniuk H, Cudzilo S. Thermal decomposition of PTFE in the presence of silicon, calcium silicide, ferrosilicon and iron. J Therm Anal Calorim. 2003;74:569–74.
van der Walt IJ, Neomagus HWJP, Nel JT, Bruinsma OSL, Crouse PL. A kinetic expression for the pyrolytic decomposition of polytetrafluoroethylene. J Fluorine Chem. 2008;129:314–8.
Garcia AN, Viciano N, Font R. Products obtained in the fuel-rich combustion of PTFE at high temperature. J Anal Appl Pyrolysis. 2007;80:85–91.
Meissner E, Worolewska A, Milchert E. Technological parameters of pyrolysis of waste polytetrafluoroethylene. Polym Degrad Stab. 2004;83:163–72.
Ebrachimi-Kahrizsangi R, Abbasi MH. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferrous Met Soc China. 2008;18:217–21.
Lipskis AA, Kviklis AV, Lipskene AM, Machynlis AN. Calculation of kinetic parameters of the thermal decomposition of polymers. Polym Sci USSR. 1976;18:489–95.
Budrugeac P, Segal E. Thermooxidative degradation of an unsaturated polyester resin. J Therm Anal. 1997;49:183–91.
Chiriac M, Rosu A, Dumitras M, Odochian L. Some aspects of the thermokinetic nonisothermal study on the thermooxidative degradation polytetrafluotoethylene containing additives. Iranian Ploym J. 2003;12:165–70.
Howell B, Zhang J. Thermal degradation of vinylidene chloride/vinyl chloride copolymers in the presence of N-substituted maleimides. J Therm Anal Calorim. 2006;83:83–6.
Vyazovkin S. Model-free kinetics. Staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Vlaev LT, Georgieva VG, Genieva SD. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Calorim. 2007;88(3):805–12.
Ozawa T. A new method of analyzing thermogravimetric data. Bul Chem Soc Japan. 1965;38:1881–6.
Paik P, Kar KK. Kinetics of thermal degradation and estimation of lifetime for polypropylene particle: effect of particle size. Polym Degrad Stab. 2008:93:24–35
Flynn JH. The ‘Temperature Integral’—its use and abuse. Thermochim Acta. 1997;300:83–92.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Cadenato A, Morancho JM, Fernandez-Francos X, Salla JM, Ramis X. Comparative kinetic study of the non-isothermal thermal curing of bis-GMA/TEGDMA systems. J Therm Anal Calorim. 2007;89:233–44.
Popescu C. Integral method to analyze the kinetics of heterogeneous reactions under nonisothermal conditions A variant on the Ozawa-Flynn-Wall method. Thermochim Acta. 1996;285:309–23.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Liqing L, Donghua C. Application of iso-temperature method of multiple rate to kinetic analysis. J Therm Anal Calorim. 2004;78:283–93.
Heide K, Höland W, Gölker H, Seyfarth K, Müller B, Sauer R. Die bestimmung kinetischer parameter endothermer zersetzungsreaktionen unter nicht-isothermen bedingungen. Thermochim Acta. 1975;13:365–78.
Zhang JJ, Ge LG, Zha XL, Dai YJ, Chen HL, Mo LP. Thermal decomposition kinetics of the Zn(II) complex with norfloxacin in static air atmosphere. J Therm Anal Calorim. 1999;58:269–78.
Horowitz HH, Metzger G. A new analysis of thermogravimetric traces. Anal Chem. 1963;35:1464–8.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature (London). 1964;201:68–9.
Madhusudanan PM, Krishnan K, Ninan KN. New approximation for the p(x) function in the evaluation of non-isothermal kinetic data. Thermochim Acta. 1986;97:189–201.
Madhusudanan PM, Krishnan K, Ninan KN. New equations for kinetic analysis of nonisothermal reactions. Thermochim Acta. 1993;221:13–21.
Tang W, Liu Y, Zhang H, Wang C. New approximate formula for Arrhenius temperature integral. Thermochim Acta. 2003;408:39–43.
Wanjun T, Yuwen L, Hen Z, Zhiyong W, Cunxin W. New temperature integral approximate formula for non-isothermal kinetic analysis. J Therm Anal Calorim. 2003;74:309–15.
Budrugeac P, Segal E. Some methodological problems concerning nonisothermal kinetic analysis of heterogeneous solid-gas reactions. Int J Chem Kinet. 2001;33:564–73.
Gao Z, Amasaki I, Nakada M. A description of kinetics of thermal decomposition of calcium oxalate monohydrate by means of the accommodated Rn model. Thermochim Acta. 2002;385:95–103.
Chunxiu G, Yufang S, Donghua C. Comparative method to evaluate reliable kinetic triplets of thermal decomposition reactions. J Therm Anal Calorim. 2006;76:203–16.
Su T-T, Jiang H, Gong H. Thermal stabilities and the thermal degradation kinetics of poly(ε-Caprolactone). Polymer-Plastics Technol Eng. 2008;47:398–403.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Cordes HF. The preexponential factors for solid-state thermal decomposition. J Phys Chem. 1968;72:2185–9.
Criado JM, Pérez-Maqueda LA, Sánchez-Jiménez PE. Dependence of the preexponential factor on temperature. Errors in the activation energies calculated by assuming that A is constant. J Therm Anal Calorim. 2005;82:671–5.
Nikolaev AV, Logvinenko VA, Gorbatchov VM, Miachina LI. On the correction of some models regarding the relationship of the kinetic parameters from the conditions of the nonisothermic experiment. Thermal analysis. In: Proceedings of the fourth ICTA, Budapest, Hungary, vol. 1; 1974. p. 47–55.
Zmijevski T, Pysiak J. Compensation effect in thermal dissociation processes. Thermal analysis. In: Proceedings of the fourth ICTA, Budapest, Hungary, vol. 1; 1974. p. 205–11.
Koga N, Tanaka H. A kinetic compensation effect established for the thermal decomposition of a solid. J Therm Anal Calorim. 1991;37:347–63.
Turmanova SCh, Genieva SD, Dimitrova AS, Vlaev LT. Non-isothermal degradation kinetics of filled with rice husk ash polypropene composites. Express Polym Lett. 2008;2:133–46.
Dias DS, Crespi MS, Ribeiro CA, Fernandes JLS, Cerqueira HMG. Application of nonisothermal cure kinetics on the interaction of poly(ethylene terephthalate)—Alkyd resin paints. J Therm Anal Calorim. 2008;91:409–12.
Dias DS, Crespi MS, Ribeiro CA. Non-isothermal decomposition kinetics of the interaction of poly(ethylene terephthalate) with alkyd varnish. J Therm Anal Calorim. 2008;94:539–43.
Ruvolo-Filho A, Curti PS. Chemical kinetic model and thermodynamic compensation effect of alkaline hydrolysis of waste poly(ethylene terephthalate) in nonaqueous ethylene glycol solution. Ind. Eng Chem Res. 2006;45:7985–96.
Frost AA, Pearson RG. Kinetics and mechanism of chemical reactions. New York: John Wiley and Sons; 1961.
Sokolskii DV, Druz VA. Vvedenie v teoriy geterogenogo kataliza. Moscow: Vischaya Shkola; 1981. (in Russian).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genieva, S.D., Vlaev, L.T. & Atanassov, A.N. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim 99, 551–561 (2010). https://doi.org/10.1007/s10973-009-0191-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0191-4