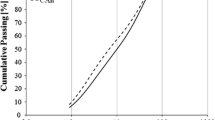
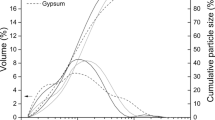
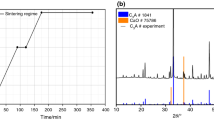
Abstract
Calcium sulfoaluminate (CSA) cements, which represent a CO2-friendly alternative to conventional Portland cements, are produced by blending CSA clinker with gypsum and/or anhydrite. The hydration kinetics and the hydrated phase assemblages of the main hydraulic phase ye’elimite (calcium sulfoaluminate) with calcium sulfate were studied by isothermal conduction calorimetry, thermogravimetric analysis, X-ray diffraction analysis and thermodynamic modelling. Two calcium sulfates with different reactivities (gypsum and anhydrite) were applied. It was found that the pure phase without any calcium sulfate addition exhibits very slow hydration kinetics during the first 10 h. The hydration can be accelerated by the addition of calcium sulfate or (less effective) by increasing the pH of the aqueous phase. The amount of the calcium sulfate determines the ratio between the hydration products ettringite, monosulfate and amorphous aluminium hydroxide. The reactivity of the added calcium sulfate determines the early hydration kinetics. It was found that the more reactive gypsum was better suited to control the hydration behaviour of ye’elimite.









Similar content being viewed by others
Notes
Cement notation: A = Al2O3, C = CaO, H = H2O, \( \overline{\text{S}} = {\text{SO}}_{3} . \)
References
Gartner E. Industrially interesting approaches to “low-CO2” cements. Cem Concr Res. 2004;34:1489–98.
Su M, Kurdowski W, Sorrentino F. Development in non-Portland cements. In: Ninth international congress on the chemistry of cements, New Delhi, India, Nov. 23–28, 1992, vol. I, p. 317–54.
Wang Y, Su M. The third cement series in China. World Cem. 1994;25(8):6–10.
Wang YM, Su MZ, Zhang L. Sulphoaluminate cement. China: Peking University Press; 1999, ISBN 7-5639-0819-6.
Zhang L, Su M, Wang Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv Cem Res. 1999;11:15–21.
Zhang L. Microstructure and performance of calcium sulfoaluminate cements. PhD thesis, University of Aberdeen; 2000.
Glasser FP, Zhang L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem Concr Res. 2001;21:1881–6.
Majling J, Znášik R, Gabrišová A, Svetík Š. The influence of anhydrite reactivity upon hydration of calcium sulphoaluminate cement clinker. Thermochim Acta. 1985;92:349–52.
Kaprálik I, Hanic F. Phase relation in the subsystem \( {\text{C}}_{ 4} {\text{A}}_{ 3} \overline{\text{S}} \)-\( {\text{C}}\overline{\text{S}} {\text{H}}_{2} \)-CH-H2O of the system CaO-Al2O3-\( {\text{C}}\overline{\text{S}} \)-H2O referred to hydration of calcium sulphoaluminate cement. Cem Concr Res. 1989;19:89–102.
Hanic F, Kaprálik I, Gabrisová A. 1989 Mechanism of hydration reactions in the system \( {\text{C}}_{ 4} {\text{A}}_{ 3} \overline{\text{S}} \)-\( {\text{C}}\overline{\text{S}} \)-CaO-H2O referred to hydration of sulphoaluminate cements. Cem Concr Res. 1989;19:671–82.
Sahu S, Havlica J, Tomková V, Majling J. Hydration behaviour of sulphoaluminate belite cement in the presence of various calcium sulphates. Thermochim Acta. 1991;175:45–52.
Palou MT, Majling J. Hydration in the system \( {\text{C}}_{ 4} {\text{A}}_{ 3} \overline{\text{S}} \)-\( {\text{C}}\overline{\text{S}} {\text{H}}_{ 2} \)-CH. J Therm Anal. 1996;46:557–63.
Péra J, Ambroise J, Holard E, Beauvent G. Influence of the type of calcium sulfate on the properties of calcium sulfoaluminate cement. In: Eleventh international congress on the chemistry of cements, Durban, South Africa, May 11–16, 2003, vol. 3, p. 1129–35.
Alaoui A, Nguyen VH, Divet L, Feraille A, Le Roy R. Experimental studies of hydration mechanisms of sulfoaluminate clinker. In: Twelve international congress on the chemistry of cement, Montreal, Canada, July 8–13, 2007, paper W3-11.6, 12.
Winnefeld F, Barlag S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. ZKG Int. (in press).
Winnefeld F, Lothenbach B. Hydration of calcium sulfoaluminate cements—experimental findings and thermodynamic modelling. Cem Concr Res. (in press). doi:10.1016/j.cemconres.2009.08.014.
Wang L, Glasser FP. Hydration of calcium sulphoaluminate cements. Adv Cem Res. 1996;8:127–34.
Zhang L, Glasser FP. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv Cem Res. 2002;14:141–55.
Zhang L, Glasser FP. Investigation of the microstructure and carbonation of CSA-based concretes removed from service. Cem Concr Res. 2005;35:252–60.
Odler I. Special inorganic cements. 3rd ed. London: E & FN Spon; 2000.
Lura P, Winnefeld F, Klemm S. Simultaneous measurements of heat of hydration and chemical shrinkage on hardening cement pastes. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0586-2.
Franke B. Bestimmung von Calciumoxid und Calciumhydroxid neben wasserfreiem und wasserhaltigem Calciumsilicat. Z anorg allg Chem. 1941;247:180–4.
Wadsö L. Applications of an eight-channel isothermal conduction calorimeter for cement hydration studies. Cem Int. 2005;5:94–101.
Available at http://gems.web.psi.ch, version 2.2.4 rc7. Accessed July 25, 2008.
Available at www.empa.ch/cemdata, version cemdata07.2. Accessed August 26, 2008.
Lothenbach B, Winnefeld F. Thermodynamic modelling of the hydration of Portland cement. Cem Concr Res. 2006;36:209–26.
Matschei T, Lothenbach B, Glasser FP. Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O. Cem Concr Res. 2007;37:1379–410.
Doval M, Palou M, Kovár V. Heat evolution and mechanism of hydration in CaO-Al2O3-SO3 system. Ceram Silik. 2005;49:104–8.
Guan B, Lou W, Ye Q, Fu H, Wu Z. Calorimetric study of calcium aluminate cement blended with flue gas desulfurization gypsum. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0107-3.
Gastaldi D, Boccaleri E, Canonico F, Bianchi M. The use of Raman spectroscopy as a versatile characterization tool for calcium sulphoaluminate cements: a compositional study. J Mater Sci. 2007;42:8426–32.
Acknowledgements
The authors express their thanks to Empa’s lab team for their assistance in the experimental work. Dr. Barbara Lothenbach and Dr. Laure Pelletier are acknowledged for the fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winnefeld, F., Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J Therm Anal Calorim 101, 949–957 (2010). https://doi.org/10.1007/s10973-009-0582-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0582-6