Abstract
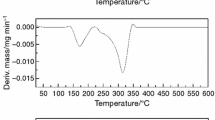
Data on the thermal stability of drugs was required to obtain information for handling, storage, shelf life and usage. In this study, the thermal stability of two nonsteroidal anti-inflammatory drugs (NSAIDs) was determined by differential scanning calorimetry (DSC) and simultaneous thermogravimetery/differential thermal analysis (TG/DTA) techniques. The results of TG analysis revealed that the main thermal degradation for the naproxen and celecoxib occurs in the temperature ranges of 196–300 and 245–359 °C, respectively. The TG/DTA analysis of compounds indicates that naproxen melts (at about 158.1 °C) before it decomposes. However, the thermal decomposition of the celecoxib started about 185 °C after its melting. The influence of the heating rate (5, 10, 15, and 20 °C min−1) on the DSC behavior of the both drug samples was verified. The results showed that, as the heating rate was increased, decomposition temperatures of the compounds were increased. Also, the kinetic parameters such as activation energy and frequency factor for the compounds were obtained from the DSC data by non-isothermal methods proposed by ASTM E696 and Ozawa. Based on the values of activation energy obtained by various methods, the following order for the thermal stability was noticed: naproxen > celecoxib. Finally, the values of ΔS #, ΔH #, and ΔG # of their decomposition reaction were calculated.


Similar content being viewed by others
References
Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, et al. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase. Eur J Biochem. 1994;221:889–97.
Ravikumar K, Rajan SS, Pattabhi V, Gabe EJ. Structure of naproxen, C14H14O3. Acta Crystallogr. 1985;C41:280–2.
Tseng PH, Wang YC, Weng SC, Weng JR, Chen CS, Brueggemeier RW, et al. Overcoming trastuzumab resistance in HER2-overexpressing breast cancer cells by using a novel celecoxib-derived PDK-1 inhibitor. Mol Pharmacol. 2006;70:1534–41.
Li J, Zhu J, Melvin WS, Bekaii-Saab TS, Chen CS, Muscarella P. A structurally optimized celecoxib derivative inhibits human pancreatic cancer cell growth. J Gastrointest Surg. 2006;10:207–14.
Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase associated lipocalin as a survival factor. Biochem J. 2005;391:441–8.
Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–18.
Kulp SK, Yang YT, Hung CC, Chen KF, Lai JP, Tseng PH, et al. 3-Phosphoinositide-dependent protein kinase-1/Akt signaling represents a major cyclooxygenase-2-independent target for celecoxib in prostate cancer cells. Cancer Res. 2004;64:1444–51.
Barbas R, Prohens R, Puigjaner C. A new polymorph of norfloxacin. J Therm Anal Calorim. 2007;89:687–92.
Pentak D, Sułkowski WW, Sułkowska A. Calorimetric and EPR studies of the thermotropic phase behavior of phospholipid membranes. J Therm Anal Calorim. 2008;93:471–7.
Picker-Freyer KM. An insight into the process of tablet formation of microcrystalline cellulose. J Therm Anal Calorim. 2007;89:745–8.
Santos AFO, Basílio ID Jr, de Souza FS, Medeiros AFD, Pinto MF, de Santana DP, et al. Application of thermal analysis in study of binary mixtures with metformin. J Therm Anal Calorim. 2008;93:361–4.
Michalik K, Drzazga Z, Michnik A. Calorimetric characterization of 2′,3′-dideoxyinosine water solution. J Therm Anal Calorim. 2008;93:521–6.
Pourmortazavi SM, Hajimirsadeghi SS, Hosseini SG. Characterization of the aluminum/potassium chlorate mixtures by simultaneous thermogravimetry-differential thermal analysis. J Therm Anal Calorim. 2006;84:557–61.
Lever SD, Papadaki M. Study of condition-dependent decomposition reactions: Part I. The thermal behaviour and decomposition of 2-nitrobenzoyl chloride. J Hazard Mater. 2004;115:91–100.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Tech. 2008;180:2093–102.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;141:322–6.
Kohsari I, Pourmortazavi SM, Hajimirsadeghi SS. Non-isothermal kinetic study of the thermal decomposition of diaminoglyoxime and diaminofurazan. J Therm Anal Calorim. 2007;89:543–6.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244–51.
Lehmann B, Karger-Kocsis J. Isothermal and non-isothermal crystallisation kinetics of pCBT and PBT. J Therm Anal Calorim. 2009;95:221.
Azimfar F, Kohsari I, Pourmortazavi SM. Investigation on decomposition kinetic and thermal stability of metallocene catalysts. J Inorg Organomet Polym Mater. 2009;19:181.
Wendlandt WW. Thermal analysis. 3rd ed. New York: Wiley; 1986. p. 58, 70.
Fathollahi M, Pourmortazavi SM, Hosseini SG. Particle size effects on thermal decomposition of energetic material. J Energ Mater. 2008;26:52.
ASTM E698-05. Standard test method for Arrhenius kinetic constants for thermally unstable materials. doi:10.1520/E0698-05.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Eslami A, Hosseini SG, Pourmortazavi SM. Thermoanalytical investigation on some boron-fuelled binary pyrotechnic systems. Fuel. 2008;87:3339–43.
Pourmortazavi SM, Kohsari I, Teimouri MB, Hajimirsadeghi SS. Thermal behaviour kinetic study of the dihydroglyoxime and dichloroglyoxime. Mater Lett. 2007;61:4670.
Criado JM, Perez-Maqueda LA, Sanchez-Jimenez PE. Dependence of the preexponential factor on temperature. J Therm Anal Calorim. 2005;82:671–5.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141–4.
Krabbendam-LaHaye ELM, de Klerk WPC, Krämer RE. The kinetic behaviour and thermal stability of commercially available explosives. J Therm Anal Calorim. 2005;80:495.
Lerdkanchanaporn S, Dollimore D. A thermal analysis study of ibuprofen. J Therm Anal Calorim. 1997;49:879.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sovizi, M.R. Thermal behavior of drugs. J Therm Anal Calorim 102, 285–289 (2010). https://doi.org/10.1007/s10973-009-0668-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0668-1