Abstract
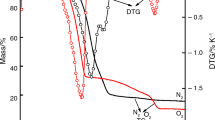
Detailed investigation on the thermal behaviour of hexaamminenickel(II) chloride and hexaamminenickel(II) bromide has been carried out by means of simultaneous TG/DTA coupled online with mass spectroscopy (TG-MS) and temperature-resolved X-ray diffraction (TR-XRD). Evolved gas analyses by TG-MS revealed the presence of NH2, NH, N2 and H2 fragments in addition to ammonia during the deamination process. These transient species resulted due to the fragmentation of the evolved ammonia during pyrolysis. The intermediates formed during the thermal deamination stages were monitored by in situ TR-XRD. The final product of the decomposition was found to be nano size metallic nickel in both cases. Morphology of the complexes, intermediates and the residue formed at various decomposition stages was analysed by scanning electron microscope (SEM). Kinetic analyses using isoconversional method for deamination and dehalogenation reaction show that the activation energies vary with the extent of conversion, indicating the multi-step nature of these solid state decomposition reactions.










Similar content being viewed by others
References
Wendlandt WW, Smith JP. Thermal properties of transition metal amine complexes. Amsterdam: Elsevier; 1967.
Mathew S, Nair CGR, Ninan KN. Thermal decomposition kinetics: kinetics and mechanism of thermal decomposition of bis(ethylenediamine)copper(II) halide monohydrate. Thermochim Acta. 1991;181:253–68.
Singh G, Pandey DK. Studies on energetic compounds: kinetics of thermal decomposition of nitrate complexes of some transition metals with propylenediamine. Combust Flame. 2003;135:135–43.
Mathew S, Nair CGR, Ninan KN. Thermal decomposition kinetics: kinetics and mechanism of thermal decomposition of tetraamminecopper(II) sulphate monohydrate. Thermochim Acta. 1989;144:33–43.
Madara’sz J. Evolved gas analyses on a mixed valence copper(I, II) complex salt with thiosulfate and ammonia by in situ TG-EGA-FTIR and TG/DTA-EGA-MS. J Therm Anal Calorim. 2009;97:111–6.
Sanders JP, Gallagher PK. Kinetic analysis of complex decomposition reactions using evolved gas analysis. J Therm Anal Calorim. 2009;96:805–11.
Mathew S, Eisenreich N, Engel W. Thermal analysis using X-ray diffractometry for the investigations of the solid state reaction of ammonium nitrate and copper oxide. Thermochim Acta. 1995;269/270:475–89.
Nair PS, Scholes GD. Thermal decomposition of single source precursors and the shape evolution of CdS and CdSe nanocrystals. J Mater Chem. 2006;16:467–73.
Navaladian S, Viswanathan B, Viswanath RP, Varadarajan TK. Thermal decomposition as a route for silver nanoparticles. Nanoscale Res Lett. 2007;2:44–8.
Li X, Zhang X, Li Z, Qian Y. Synthesis and characteristics of NiO nanoparticles by thermal decomposition of nickel dimethylglyoximate rods. Solid State Commun. 2006;137:581–4.
Chen Y, Peng D, Lin D, Luo X. Preparation and magnetic properties of nickel nano particles via thermal decomposition of nickel organometallic precursors in alkylamine. Nanotechnology. 2007;18:505703–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1996;4:323–8.
Friedman HL. New methods for evaluating kinetic parameters from thermal analysis data. J Polym Sci B. 1969;7:41–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Research report of Chiba Institute Technology. 1971;16:22–31.
Brauer G. Hand book of preparative inorganic chemistry. 2nd ed. New York: Academic Press; 1965.
Vogel AG. Text book of quantitative inorganic analysis. 4th ed. London, UK: Longmann; 1978.
Badrinarayanan P, Zheng W, Simon SL. Isoconversional analysis of the glass transition. Thermochim Acta. 2008;468:87–93.
Curtis SD, Kubiak M, Kurdziel K, Materazzi S, Vecchio S. Crystal structure and thermoanalytical study of a cadmium(II) complex with 1-allylimidazole. J Anal Appl Pyrolysis. 2010;87:175–9.
Cai JM, Bi LS. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J Therm Anal Calorim. 2009;98:325–30.
Su TT, Zhai YC, Jiang H, Gong H. Studies on the thermal decomposition kinetics and mechanism of ammonium niobium oxalate. J Therm Anal Calorim. 2009;98:449–55.
Tanaka N, Kagawa M, Kamada M. The thermal decomposition of hexaamminenickel(II) complexes. Bull Chem Soc Jpn. 1968;41:2908–13.
Madarasz J, Bombicz P, Matyas C, Reti F, Kiss G, Pokol G. Comparative evolved gas analytical and structural study on trans-diammine-bis(nitrito)-palladium(II) and platinum(II) by TG/DTA-MS, TG-FTIR and single crystal X-ray diffraction. Thermochim Acta. 2009;490:51–9.
Mikuli AM, Hetmanczyk J, Mikuli E, Hetmanczyk L. Thermal behaviour of polycrystalline [Ba(H2O)3](ClO4)2 and [Ba(NH3)4](ClO4)2. Thermochim Acta. 2009;487:43–8.
Leineweber A, Jacobs H. Preparation and crystal structure of Ni(NH3)2Cl2 and of two modifications of Ni(NH3)2Br2 and Ni(NH3)2I2. J Solid State Chem. 2000;152:381–7.
Li C, Shuford KL, Chen M, Lee EJ, Cho SO. A facile polyol route to uniform gold octahedra with tailarable size and their optical properties. ACS Nano. 2008;9:1760–9.
Padhi SK. Solid state kinetics of thermal release of pyridine and morphological study of [Ni(ampy)2(NO3)2]; ampy = 2-picolylamine. Thermochim Acta. 2006;448:1–6.
Green M, O’Brien P. The preparation of organically functionalized chromium and nickel nanoparticles. Chem Commun. 2001;1912–3.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. Int J Chem Kinet. 1996;28:95–101.
Millan A, Clemente RR, Veintemillas S, Spinner B. Decomposition and synthesis of NiCl2 ammoniate salts: an optical microscopy study. J Chem Soc Faraday Trans. 1997;93(18):3363–9.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Acknowledgements
The authors are grateful to the Sophisticated Test and Instrumentation Centre (STIC), Cochin, for recording TR-XRD patterns.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rejitha, K.S., Ichikawa, T. & Mathew, S. Thermal decomposition studies of [Ni(NH3)6]X2 (X = Cl, Br) in the solid state using TG-MS and TR-XRD. J Therm Anal Calorim 103, 515–523 (2011). https://doi.org/10.1007/s10973-010-1054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1054-8