Abstract
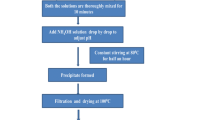
In this study, lanthanum, cerium, and praseodymium orthophosphates were synthesized by the modified Pechini method. The compounds were analyzed using XRD, TG/DSC, FTIR methods, and the isothermal nitrogen adsorption technique. The results showed that mesoporous and nanocrystalline powders can be synthesized by this method. Moreover, due to the limited formation of lanthanide polyphosphates on the surface of the powders the modified Pechini method allows better control of the compound stoichiometry in comparison with the commonly used method of phosphates precipitation from solutions rich in H3PO4.







Similar content being viewed by others
References
Mooney RCI. X-ray diffraction study of cerous phosphate and related crystals.;1. Hexagonal modification. Acta Cryst. 1950;3:337–40.
Mooney RCI. Crystal structures of a series of rare earth phosphates. J Chem Phys. 1948;16:1003.
Ushakov SV, Helean KB, Navrotsky A, Boatner LA. Thermochemistry of rare-earth orthophosphates. J Mater Res. 2001;16:2623–33.
Kijkowska R. Thermal decomposition of lanthanide orthophosphates synthesized through crystallisation from phosphoric acid solution. Thermochim Acta. 2003;404:81–8.
Hikichi Y, Nomura T. Melting temperatures of monazite and xenotime. J Am Ceram Soc. 1987;70:C252–3.
Hikichi Y, Nomura T, Tanimura Y, Suzuki S, Miyamoto M. Sintering and properties of monazite-type CePO4. J Am Ceram Soc. 1990;73:3594–6.
Kuo DH, Kriven WH, Macikn TJ. Control of interfacial properties through fiber coatings: monazite coatings in oxide-oxide composites. J Am Ceram Soc. 1997;80:2987–96.
Lewis MH, Tye A, Butler EG, Doleman PA. Oxide CMCs: interphase synthesis and novel fibre development. J Eur Ceram Soc. 2000;20:639–44.
Davis JB, Marshall DB, Morgan PED. Monazite-containing oxide/oxide composites. J Eur Ceram Soc. 2000;20:583–7.
Davis JB, Marshall DB, Oka KS, Housley RM, Morgan PED. Ceramic composites for thermal protection systems. Compos A. 1999;30:483–8.
Boakye EE, Mogilevsky P, Parthasarathy TA, Hay RS, Welter J, Kerans RJ. Monazite coatings on SiC fibers I: fiber strength and thermal stability. J Am Ceram Soc. 2006;89:3475–80.
Boakye EE, Hay RS, Petry MD. Continuous coating of oxide fiber tows using liquid precursors: monazite coatings on Nextel 720 (TM). J Am Ceram Soc. 1999;82:2321–31.
Clavier N, Dacheux N, Podor R. Synthesis, characterization, sintering, and leaching of beta-TUPD/monazite radwaste matrices. Inorg Chem. 2006;45:220–9.
Podor R, Cuney M, Trung CN. Experimental study of the solid solution between monazite-(La) and (Ca0.5U0.5)PO4 at 780 °C and 200 MPa. Am Mineral. 1995;80:1261–8.
Kitaev DB, Volkov YF, Orlova AI. Orthophosphates of tetravalent Ce, Th, U, Np, and Pu with the monazite Structure. Radiochem. 2004;46:211–7.
Bakker K, Hein H, Konings RJM, van der Laan RR, Matzke HJ, van Vlaanderen P. Thermophysical property measurements and ion-implantation studies on CePO4. J Nucl Mater. 1998;252:228–34.
Kitamura N, Amezawa K, Tomii Y, Hanada T, Yamamoto N, Omata T, Otsuka-Yao-Matsuo S. Electrical conduction properties of Sr-doped LaPO4 and CePO4 under oxidizing and reducing conditions. J Electrochem Soc. 2005;152:A658–63.
Gallini S, Hansel M, Norby T, Colomer MT, Jurado JR. Impedance spectroscopy and proton transport number measurements on Sr-substituted LaPO4 prepared by combustion synthesis. Solid State Ionics. 2003;162–163:167–73.
Bregiroux D, Audubert F, Charpentier T, Sakellariou D, Bernache-Assolant D. Solid-state synthesis of monazite-type compounds LnPO4 (Ln=La to Gd). Solid State Sci. 2007;9:432–9.
Karpowich L, Wilcke S, Yu R, Harley G, Reimer JA, De Jonghe LC. Synthesis and characterization of mixed-morphology CePO4 nanoparticles. J Solid State Chem. 2007;180:840–6.
Belina P, Myskova V, Sulcova P. Comparison of the crystallization and solid state reaction methods for the preparation of rare-earth orthophosphates. J Therm Anal Calorim. 2009;96:949–54.
Fang YP, Xu AW, Song RQ, Zhang HX, You LP, Yu JC, Liu HQ. Systematic synthesis and characterization of single-crystal lanthanide orthophosphate nanowires. J Am Chem Soc. 2003;125:16025–34.
Zhang Y, Guan H. The growth of lanthanum phosphate (rhabdophane) nanofibers via the hydrothermal method. Mater Res Bull. 2005;40:1536–43.
Pechini MP. US Patent 3330697, 11 July 1967.
Narendar Y, Messing GL. Mechanisms of phase separation in gel-based synthesis of multicomponent metal oxides. Catal Today. 1997;35:247–68.
Klug HP, Alexander LE. X-ray diffraction procedures for polycrystalline and amorphous materials. New York: Wiley & Sons; 1974.
Kijkowska R, Cholewka E, Duszak B. X-ray diffraction and Ir-absorption characteristics of lanthanide orthophosphates obtained by crystallisation from phosphoric acid solution. J Mater Sci. 2003;38:223–8.
Lucas S, Champion E, Bregiroux D, Bernache-Assolant D, Audubert F. Rare earth phosphate powders RePO4·nH2O (Re = La, Ce or Y) - Part I. Synthesis and characterization. J Solid State Chem. 2004;177:1302–11.
Assaaoudi H, Ennaciri A, Rulmont A. Vibrational spectra of hydrated rare earth orthophosphates. Vib Spectr. 2001;25:81–90.
Matraszek A, Szczygieł I. Modified Pechini synthesis of Na3Ce(PO4)2 and thermochemistry of its phase transition. J Therm Anal Calorim. 2008;93:689–92.
Corbridge DEC, Lowe EJ. The infra red spectra of some inorganic phosphorus compounds. J Chem Soc. 1954;B15:493–502.
Hezel A, Ross SD. Forbidden transitions in the infra-red spectra of tetrahedral anions—III. Spectra-structure correlations in perchlorates, sulphates and phosphates of the formula MXO4. Spectrochim Acta. 1961;22:1949–61.
Lasarev A, Mirgorodsii A. Ignatiev I. Vibrational spectra of complex oxides. Leningrad: Nauka; 1975.
Szczygiel I, Macalik L, Radominska E, Znamierowska T, Maczka M, Godlewska P, Hanuza J. Luminescence, electronic absorption and vibrational IR and Raman studies of binary and ternary cerium ortho-, pyro- and meta-phosphates doped with Pr3+ ions. Opt Mater. 2007;29:1192–205.
Nahdi K, Ferid M. Malika trabelsi ayadi. Thermal dehydratation of CeP3O3·3H2O by controlled rate thermal analysis. J Therm Anal Calorim. 2009;96:455–61.
Sing KSW, Everest DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (recommendations 1984). Pure Appl Chem. 1985;57:603–19.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matraszek, A., Radominska, E. & Szczygiel, I. Modified Pechini synthesis of La, Ce, and Pr orthophosphates and characterization of obtained powders. J Therm Anal Calorim 103, 813–819 (2011). https://doi.org/10.1007/s10973-010-1063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1063-7