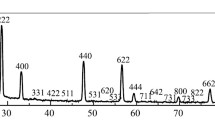
Abstract
Characterization, thermal stability and thermal decomposition of light trivalent lanthanide fumarates, as well as, the thermal behaviour of fumaric acid and its sodium salt were investigated employing simultaneous thermogravimetry and differential thermal analysis, differential scanning calorimetry, Fourier transform infrared spectroscopy (FTIR), TG–FTIR techniques, elemental analysis and complexometry. On heating, sublimation of fumaric acid is observed, while the thermal decomposition of the sodium fumarate occurs with the formation of a mixture of sodium carbonate and carbonaceous residue. The thermal decomposition of light trivalent lanthanide fumarates occurs in consecutive and/or overlapping steps with the formation of the respective oxides: CeO2, Pr6O11, and Ln2O3 (Ln = La, Nd, Sm, Eu, Gd).





Similar content being viewed by others
References
Jansen J, Melchels Ferry PW, Grijpma DW, Feijen J. Acid monoethyl esther-functionalized poly(d, l-lactide)/N-vinyl-2-pyrrolidone resins for the preparation of tissue engineering scaffolds by stereolithography. Biomacromolecules. 2009;10:214–20.
Aleksandrovic V, Djonlagic J. Synthesis and characterization of thermoplastic copolyester elastomers modified with fumaric moieties. J Serb Chem Soc. 2001;66:139–52.
Ohnishi M, Uno T, Kubo M, Itoh T. Synthesis and radical polymerization of dissymmetric fumarates with alkoxyethyl, bulky siloxy groups. J Polym Sci A Polym Chem. 2009;47:420–33.
Zhu WH, Wang ZM, Gao S. Two 3D porous lanthanide–fumarate–oxalate frameworks exhibiting framework dynamics and luminescent change upon reversible de- and rehydration. Inorg Chem. 2007;46:1337–42.
Zhu WH, Wang ZM, Gao S. A 3D porous lanthanide–fumarate framework with water hexamer occupied cavities, exhibiting a reversible dehydration and rehydration procedure. Dalton Trans. 2003;6:765–8.
Mac Ginn MJ, Wheeler BR, Galwey AK. Thermal decomposition of nickel fumarate. Trans Faraday Soc. 1970;66:1809–16.
Sevost’yanov VP, Dvornikova LM. Thermal decomposition of gadolinium fumarate and succinate. Izv Vyss Uchebn Zaved Khim Khim Tekhnol. 1971;14:1771–3.
Sevost’yanov VP, Dvornikova LM. Thermal decomposition of ytterbium fumarate and succinate. Zhur Neorg Khim. 1972;17:2884–7.
Bassi PS, Randhawa BS, Khajuria CM, Kaur S. Comparative study of the thermal analyses of some transition metal(II) maleates and fumarates. J Therm Anal. 1987;32:569–77.
Allan JR, Bonner JG, Bowley HJ, Gerrard DL, Hoey S. Thermal studies on fumaric acid and crotonic acid compounds of cobalt(II) and nickel(II). Thermochim Acta. 1989;141:227–33.
Ionashiro M, Graner CAF, Zuanon Netto J. Complexometric titration of lanthanides and yttrium. Ecl Quim. 1983;8:29–32.
Socrates G. Infrared characteristic group frequencies. 2nd ed. New York: Wiley; 1994. p. 91, 236–7.
Silverstein RM, Webster FX. Spectrometric identification of organic compounds. 6th ed. New York: Wiley; 1998. p. 92, 93, 96, 97.
Lewandowski W, Baranska H. Vibrational and electronic spectroscopic study of lanthanides and effect of sodium on the aromatic system of benzoic acid. J Raman Spectrosc. 1986;17:17–22.
Siqueira AB, Carvalho CT, Ionashiro EY, Bannach G, Rodrigues EC, Ionashiro M. Synthesis, characterization and thermal behaviour of solid 2-methoxybenzoates of trivalent metals. J Therm Anal Calorim. 2008;98:945–51.
Locatelli JR, Rodrigues EC, Siqueira AB, Ionashiro EY, Bannach G, Ionashiro M. Synthesis, characterization and thermal behaviour of solid-state compounds of yttrium and lanthanide benzoates. J Ther Anal Calorim. 2007;90:737–46.
Siqueira AB, Bannach G, Rodrigues EC, Carvalho CT, Ionashiro M. Solid-state 2-methoxybenzoates of light trivalent lanthanides. Synthesis, characterization and thermal behaviour. J Therm Anal Calorim. 2008;91:897–902.
Acknowledgements
The author thanks FAPESP, CNPQ Edital Universal and CAPES Brazilian agencies for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ionashiro, E.Y., Caires, F.J., Siqueira, A.B. et al. Thermal behaviour of fumaric acid, sodium fumarate and its compounds with light trivalent lanthanides in air atmosphere. J Therm Anal Calorim 108, 1183–1188 (2012). https://doi.org/10.1007/s10973-011-1660-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1660-0