Abstract
The aim of the research was obtaining and application of smectic clay modifying agent. The organophilic clay is used as nanofiller in polymer nanocomposites [1]. A microwave-assisted reaction led to obtaining N-heptaquinolinum, which is amphiphilic compound, containing both hydrophobic (alkyl and aromatic) and hydrophilic sections in its structure [2]. N-heptaquinolinum was used as a montmorillonite clay modifying agent. Modification was carried out in formulated way [3, 4]. Modification efficiency was determined by X-ray diffraction (XRD) analysis and elementary analysis. Organophilic clay (Ch7) was introduced, using the extrusion method, into polyethylene matrix in different mass relations (1.5, 3 and 5 %) [3]. The structure of obtained materials was studied by means of XRD and SEM. To evaluate potential applications thermal properties of received nanocomposites were tested with thermogravimetric analysis and differential scanning calorimetry. The thermal stability of PE/clay composites can be improved in the case of loading 1.5 and 5 mass%.
Similar content being viewed by others
Introduction
Recent years have been the age of polymer nanocomposites, in particular those based on organically modified nanoclays such as montmorillonite (MMT). Nanofillers are introduced into the polymer matrix in order to significantly improve a number of physico-chemical properties required in new fields of application. In most cases the introduction of nanofillers into the polymer results in considerable increase in the elasticity module, increased thermal stability or higher fire retardancy factor [5–7]. Increased thermal resistance of polymers is obtained as a result of the formation of surface carbonation structure i.e., a mechanically resistant barrier layer which hinders the formation and emission of gases during combustion and at the same time provides thermal insulation for the polymer. Nanofillers considerably limit heat transmission within a polymer and therefore confine open flame preventing the smouldering material from dripping [8, 9].
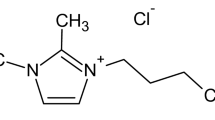
In order to obtain the above mentioned properties the highest possible level of dispersion of the nanoclay in the polymer matrix is essential. This is most often achieved by modifying the filler involving the ion exchange of the Na+ and the Ca2+ ions of the mineral by organophilic cations [10]. Resulting clay allows for the creation of nanocomposite where the polymer penetrates the layers of the organically modified filler [11]. The volume of the nanofiller being introduced into the polymer matrix ranges form 1.5 to 5 wt%, which is enough to obtain specific, high standards required of the resulting material. One of the compounds meeting the compatibilizing agent requirements, used in order to modify montmorillonite is N-heptaquinolinum.
The figure below (Fig 1) depicts the montmorillonite organophilization process. Organophilic clay was introduced, using the extrusion method, into polyethylene (PE) matrix in different mass relations (1.5, 3, and 5 %).
Experimental part
Materials
The natural clay mineral—montmorillonite used in this study was provided by the Riedel–de Haen. The surfactant used for the modification was N-heptaquinolinum, obtained in the reaction of quinolinum (Aldrich, Germany). and n-heptyl bromide (Aldrich, Germany). The modification was carried out using methanol and hydrochloric acid, (POCH, Poland). Polyethylene MALEN FABS was delivered by Basell –ORLEN Polyolefins (Plock, Poland). Compatibilizing agent—PE graft-maleic anhydrate Fusabond UB 226(DuPont) d = 0.93 g/cm³; MFR = 1.75 g/10 min.
The synthesis of modifying agent
N-heptaquinolinum was obtained in a reaction of quinolinum and n-heptyl bromide according to literature Ref. [2] and in microwave-assisted method. The microwave method allows the shortening of the reaction time from 48 h to 3 min and causes the reaction to be more efficient.
Preparation of surfactant-modified montmorillonite and nanocomposites
The ion-exchange reaction was carried out in order to prepare the organophilic montmorillonite. Montmorillonite powder with N-heptaquinolinum were later stirred vigorously in water/methanol mixture. HCl was slowly added to obtain cations of N-heptaquinolinum. The temperature of the mixture was increased from room temperature to about 343 K. The reaction continued for 5 h. After every hour small amount of sample was retrieved.
All organoclay products were washed several times until no chloride ions were detected by AgNO3 solution. Organoclay was dried for 24 h at 353 K. The initial ratio amine/clay and HCl/amine were 2 mmol/g and 2 molar ratio respectively [12] The nanocomposites of PE with modified montmorillonite (Ch7) (from 1.5 to 5 mass%) and 5 mass% of compatibilizing agent were prepared by melt intercalation process (obtaining PE-Ch7-1.5, PE-Ch7-3 and PE-Ch7-5 respectively).
Characterization techniques
Powder X-ray diffraction (XRD) patterns were recorded using a X’Pert Philips diffractometer using Cu Kα filtered radiation. (λ = 1.5418 Å) at 40 kV, 40 mA between 1° and 12° 2θ. Divergence slit size was 0.125o; scan step time 25 s.
Fracture surfaces of PE-Ch7-1.5 (PE was investigated using scanning electron microscopy (SEM) LEO Electron Microscopy Ltd, 1430 VP. Before investigation the sample was sputter coated with silver.
Differential scanning calorimetry (DSC) analyses of new materials were conducted using a Polymer Laboratories, Epson, GB. All samples were investigated under N2 with flow rate about 15 ml/min and heating rate of 10 K/min.
Thermal analyses of surfactant-modified montmorillonite and nanocomposites were carried out at a heating rate of 10 K/min under the flow of air and nitrogen from room temperature to 873 K. All measurements were performed on TA Instruments, SPT 2960 Simultaneous DSC–TGA.
Elementary analysis of the modified MMT for the determination of C and N was carried out with Vario MACRO CHN Elementary Analyzer (Analysensysteme, Germany).
Resultus and discussion
Structure analysis
In an attempt to evaluate whether the modification process occurred or not, samples of organoclay after every hour of modification were analyzed by XRD. Table 1 gives a summary of the d-spacing for both unmodified MMT and MMT after 1, 2, 3, 4 and 5 h (Ch7-1 h, Ch7-2 h, Ch7-3 h, Ch7-4 h, Ch7-5 h respectively) while Fig. 2 shows the XRD curves for all samples of investigated clays. It is clear from this data that a significant increase in the gallery spacing between the clay layers was observed for all investigated organomodified samples, compared to the unmodified MMT. Pristine MMT shows a characteristic diffraction peak at 6.23o which corresponds to a d-spacing of 1.42 nm. MMT after 2 h of modification shows that d-spacing moves to 5.17 (1.71 nm) by surfactant exchange. However, all modified samples show a much smaller and broader peak compared with pristine MMT. It can be due to the inhomogeneous distribution of the surfactant between the layers of the clay, presenting a broader range of interlayer distances depending on the extent of ion exchange [13].
The most significant distribution was observed toward lower angles in the case of the sample retrieved after 2 h of modification, it was used to prepare PE-clay nanocomposites. X-ray diffractometry was also used to evaluate the degree of interaction between the organoclay and the PE [14]. The XRD patterns for the PE and PE-based nanocomposites containing 1.5–5 mass% clay are shown in Fig. 3.The XRD curves of modified MMT and PE-Ch7 materials present a peak at the same value of 2θ. This proves that the organoclay dispersed in the PE matrix did not lose the ordered structure of lamella, keeping the same spacing [7]. It is well known that during the ion-exchange the surface of the clay can change from hydrophilic to hydrophobic, and can be used to obtain most of polymer/clay nanocomposites. But the polarity of PE probably is too low which could indicate that the compatibility of modified MMT and PE was poor and insufficient to form a nanocomposite [15, 16]. For this reason we can assume that PE-Ch7 composites are conventional microcomposites [15].
In the case of 1.5 wt% of filler the amount of clay was too low to be detected in XRD analysis. Because of that the use of electron microscopy was necessary to study the structure [17]. SEM observations of PE-Ch7-1.5 sample are shown in Fig. 4. Based on the conducted analysis of the fracture surfaces it is not possible to determine the structure of the investigated material because there is no possibility of observing significant changes in the structure.
Elementary analysis
This method was used for determining the quantity of the modifying agent absorbed by MMT during modification process [3]. For this reason the content of C was checked in neat MMT and all samples of modified MMT retrieved during modification process after different time of modification. Results of this analysis and calculation are listed in Table 2.
In view of the above mentioned results the quantity of the modifier absorbed by 1 g of MMT and percentage consumption of N-heptaquinolinum were determined.
Based on the results concerning the modifier consumption, it is apparent that the process of clay surface modification is one of saturation as the function of time of the conducted modification process. This allows for the confirmation that the procedure was conducted properly.
Elementary analysis results differ from XRD analysis in terms of indication of the potential, optimum modification time. XRD study indicates that the most appropriate time for conducting modification are 2 h, while the elementary analysis suggests that a more lengthy procedure is more suitable. In the matter of fact, as aforementioned, the surface modification process is of saturating characteristic. Consequently the more lengthy modification would result in an increase in the quantity of the modifying agent bound to the lay surface, although the increase in quantity would subside until it reached an equilibrium state in terms of the modifier bound to the clay and the modifier present in the solution. Longer duration of performing modification and, at the same time, an increased modifier overlay on the clay result in a progressing mutual chemical affinity of the modified clay surfaces, leading—after a period of 2 h—to a narrowing of interlayer spaces (1.66 nm in the case of 3 h long modification), resulting from derivative formation of MMT tiles, caused by an increased chemical affinity of modified surfaces.
Thermal properties
DSC analysis has been carried out to study first-order transitions like melting and crystallization of newly obtained materials. Results are presented in Table 3. Properties studied were the melting temperature (T m), the heat of fusion (H m) during PE melting and the degree of crystallinity (X c), where enthalpy of melting of pure crystalline PE is 279 J g−1 [18].
For all samples the increase in the melting point could have been observed due to the higher reinforcement [19] It has been observed that with an increasing amount of modified MMT, the melting peak temperature decreases till 390.9 K for nanocomposite PE-Ch7-5 with 5 mass% of organoclay. This suggests that clay platelets act as nucleating centers and favour crystallization by proving a higher level of nucleating density [20].
The obtained results clearly show that the introduction of a small amount of modified MMT into the PE matrix causes an increase of heat enthalpy, especially at 1.5 mass% concentration of loading (107.9 J g−1) and decreases slightly with further increase in clay concentration until 93.8 J g−1.
DSC analysis was complemented by thermogravimetric analysis in order to precisely determine thermal stability of obtained materials.
TGA is widely used to characterize the thermal properties of polymers and their composites, however this technique has also been adopted in order to determine thermal stability of polymer nanofillers.
Figure 5 presents the TGA curves for unmodified (MMT) and modified montmorillonite (Ch7), the sample which was selected to obtain composites. It is apparent from this study that decomposition of MMT occurs at 338–393 K and it is attributed to the volatilization of physisorbed water. In comparison with neat MMT organoclay has got less free water in the modified MMT. This is caused by N-heptaquinolinum which reduces the surface energy of the inorganic material, and converts the hydrophilic silicate surface to an organophilic one [21, 22]. The presence of organic cations increases the number of decomposition steps.
It is known that organoclay has got two opposing functions in the thermal stability of nanocomposites. The first one amounts to the barrier effect can improve the thermal stability, the other one is related to the fact that the clay is a catalyst in the decomposition of polymer matrix as a result of the Hofmann elimination reaction [3, 23, 24]. Apart from that, the clay itself can also catalyse the degradation of a polymer [25, 26]. Table 3 and Fig. 6 show the curves of pure PE and for nanocomposites containing 1.5, 3 and 5 mass% from which the data were obtained. The parameters that were of interest include the temperature at which 10 % degradation occurs, the 50 % degradation temperature and the amount of non-volatile residue that remains at 873 K, known as char [14]. Char may act as a physical barrier between the polymer medium and the superficial zone where flame combustion occurs constituting a measure of an inorganic content [3]. In the case of composites filled with 1.5 and 5 mass% the thermal degradation temperature increases in comparison with pure PE. The most significant increase in decomposition temperatures at 10 % mass loss and 5 % filler content amounted to approx. 36 K in comparison with the original polymer matrix. It is surprising that the increase in degradation temperature is not related with the increase in the amount of char. In the case of PE-Ch7-3 composite the quantity of char is the highest and in spite of that the compound displays decomposition temperatures at 10 % mass loss lower by 10 K when compared to neat PE (Table 3). Decomposition temperatures at 15 % mass loss for all investigated materials are higher than in the case of the original polymer matrix, whereas the most significant increase occurred in PE-Ch7-1.5, and the least notable in 3 mass% of filler content.
In view of the above mentioned facts our results confirm that the PE-Ch7 nanocomposites show a higher thermal stability than pure PE.
Conclusions
-
Based on the results obtained by means of XRD analysis the optimum time of modification and the impact the modifying agent has got on the silicate layer separation were determined.
-
In order to obtain the new materials based on PE, clay modified within a two hour time span and assuring the most significant layer separation was used.
-
The presence of diffraction peak in investigated new materials at the same place as signal derived from MMT-Ch7 can be the evidence that composite structure was obtained for all PE-based materials.
-
Thermal analysis of the modified clay suggests that the clay is stable within the temperature range of PE processing.
-
Thermograms of the newly obtained nanocomposites clearly indicate an increase in the degradation temperature.
-
The melting temperature as well as the transition heat increased in the case of compounds based on PE which suggests an improvement of the composite structural arrangement.
References
Pavlidou S, Papaspyrides CD. A review on polymer–layered silicate nanocomposites. Prog Polym Sci. 2008;33:1119–98.
Chigwada G, Wang D, Wilkie ChA. Polystyrene nanocomposites based on quinolinium and pyridinium surfactants. Polym Degrad Stab. 2006;91:848–55.
Olewnik E, Garman K, Czerwiński W. Thermal properties of new composites based on nanoclay, polyethylene and polypropylene. J Therm Anal Calorim. 2010;101:323–9.
Xiao W, Zhan M, Li Z. Organically modifying and modeling analysis of montmorillonites. Mater Des. 2003;24:455–62.
Ray SS, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28:1539–641.
Gopakumar TG, Lee JA, Kontopoulou M, Parent JS. Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene. Polymer. 2002;43:5483–91.
Zanetti M, Bracco P, Costa L. Thermal degradation behaviour of PE/clay nanocomposites. Polym Degrad Stab. 2004;85:657–65.
Araujo EM, Barbosa R, Morais CRS, Soledade LEB, Souza AG, Vieira MQ. Effects of organoclays on the thermal processing of PE/clay nanocomposites. J Therm Anal Calorim. 2007;90:841–8.
Leszczynska A, Njuguna J, Pielichowski K, Banerjee JR. Polymer/montmorillonite nanocomposites with improved thermal properties. Part I. Thermochim Acta. 2007;453:75–96.
Kozak M, Domka L. Adsorption of the quaternary ammonium salts on montmorillonite. J Phys Chem Solids. 2004;65:441–5.
Ding C, Jia D, He H, Guo B, Hong H. How organo-montmorillonite truly affects the structure and properties of polypropylene. Polym Test. 2005;24:94–100.
Perez-Santos A, Trujillano R, Belver C, Gil A, Vicente MA. Effect of the intercalation condition of a montmorillonite with octadecylamine. J Colloid Interf Sci. 2005;284:239–44.
García-Lopez D, Picazo O, Merino JC, Pastor JM. Polypropylene–clay nanocomposites: effect of compatibilizing agents on clay dispersion. Eur Polym J. 2003;39:945–50.
Chigwada G, Jiang DD, Wilkie CA. Polystyrene nanocomposites based on carbazole-containing surfactants. Thermochim Acta. 2005;436:113–21.
Zhao C, Qin H, Gong F, Feng M, Zhang S, Yang M. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym Degrad Stab. 2005;87:183–9.
Samyn F, Bourbigot S, Jama Ch, Bellayer S, Nazare S, Hull R, Fina A, Castrovinci A, Camino G. Characterisation of the dispersion in polymer flame retarded nanocomposite. Eur Polym J. 2008;44:1631–41.
Filho FGR, Melo TJA, Rabello MS, Silva SML. Thermal stability of nanocomposites based on polypropylene and bentonite. Polym Degrad Stab. 2005;89:383–92.
Durmus A, Woo M, Kasgoz A, Macosko CW, Tsapatsis M. Intercalated linear low density polyethylene (LLDPE)/clay nanocomposites prepared with oxidized polyethylene as a new type compatibilizer: structural, mechanical and barrier properties. Eur Polym J. 2007;43:3737–49.
Sharma SK, Nayak SK. Surface modified clay/polypropylene (PP) anocomposites: effect on physico-mechanical, thermal and morphological properties. Polym Degrad Stab. 2009;94:132–8.
Ratna D, Divekar S, Samui AB, Chakraborty BC, Banthia AK. Poly(ethylene oxide)/clay nanocomposite: thermo-mechanical properties and morphology. Polymer. 2006;47:4068–74.
Frost R, Xi Y, He H. Modification of the surfaces of Wyoming montmorillonite by the cationic surfactants alkyl trimethyl, dialkyl dimethyl and trialkyl methyl ammonium bromides. J Colloid Interf Sci. 2007;305:150–8.
Zhou L, Chena H, Jiang X, Lu F, Zhou Y, Yin W, Ji X. Modification of montmorillonite surfaces using a novel class of cationic gemini surfactants. J Colloid Interf Sci. 2009;332:16–21.
Zanetti M, Camino G, Thomann R, Mulhaupt R. Synthesis and thermal behaviour of layered silicate EVA nanocomposites. Polymer. 2001;42:4501–7.
Xie W, Gao Z, Pan WP, Hunter D, Singh A, Vaia R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem Mater. 2001;13:2979–90.
Qin H, Zhang S, Zhao C, Feng M, Yang M, Shu Z, Yang S. Thermal stability and flammability of polypropylene/montmorillonite composites. Polym Degrad Stab. 2004;85:807–13.
Zanetti M, Camino G, Reichert P, Mulhaupt R. Thermal behavior of poly(propylene) layered silicate nanocomposites. Macromol Rapid Commun. 2001;22:176–80.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Olewnik, E., Garman, K., Piechota, G. et al. Thermal properties of nanocomposites based on polyethylene and n-heptaquinolinum modified montmorillonite. J Therm Anal Calorim 110, 479–484 (2012). https://doi.org/10.1007/s10973-012-2380-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2380-9