Abstract
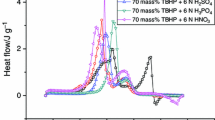
Isothermal microcalorimetry can be used to investigate the thermokinetic parameters for reactive mechanisms. Benzoyl peroxide (BPO), a typical organic peroxide, undergoes an autocatalytic reaction under isothermal decomposition. It requires intrinsically safer design of preparation, manufacturing, transportation, storage, and even disposal. The scope of this study was to describe the exothermic reaction and reaction model of BPO and mixed with benzoic acid by the thermal activity monitor III (TAM III). The results showed the isothermal kinetic parameters, such as activation energy (E a), frequency factor (A), heat of decomposition (ΔH d), and time to maximum rate under isothermal conditions (TMR iso), which were necessary and useful to insure safe storage or transportation for self-reactive substances applied in the process industries.








Similar content being viewed by others
Abbreviations
- A :
-
Reactant
- C dT/dt :
-
Rate of heat accumulation or depletion (kJ kg−1 min−1)
- dC/dt :
-
Reaction rate (mol L−1 s−1)
- dQ/dt :
-
Heat production rate (kJ kg−1 min−1)
- f(c):
-
Kinetic function depends on conversions
- g :
-
Seebeck coefficient (V K−1)
- k :
-
Reaction rate constant (min−1)
- k a :
-
Heat conductance (W K−1)
- P :
-
Thermal power or heat production rate (W = J s−1)
- Q :
-
Heat of decomposition (J)
- Q max :
-
Maximum peak power at time t (W g−1)
- R :
-
Product
- T :
-
Temperature of sample (K)
- TMR iso :
-
Time to maximum rate under isothermal conditions (hr)
- T 0 :
-
Temperature of surroundings (K)
- t :
-
Time (s)
- U :
-
Heat transfer coefficient (kJ min−1 m−2 K−1)
- v :
-
Rate of change (mol s−1)
- ΔH :
-
Enthalpy change (J g−1)
- ∆H d :
-
Heat of decomposition (J g−1)
- Φ :
-
Slope of the heat flow time curve (W = J s−1)
- τ :
-
Time constant obtained from dynamic calibration (s)
- ε :
-
Calibration constant (W V−1)
References
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2001;14:229–39.
Tseng JM, Shu CM, Yu YC. Thermal hazard simulations for methyl ethyl ketone peroxide induced by contaminants. Korean J Chem Eng. 2005;22:797–802.
Fine DH, Gray P. Explosive decomposition of solid benzoyl peroxide. Combust Flame. 1967;11:71–8.
Zaman F, Beezer AE, Mitchell JC, Clarkson Q, Elliot J, Davis AF, Willson RJ. The stability of benzoyl peroxide by isothermal microcalorimetry. Int J Pharm. 2001;277:133–7.
Bamford CH, Tipper CF. Chemical kinetics: decomposition and isomerisation of organic compounds, vol 5. New York: Elsevier; 1977.
Chervin S, Bodman GT. Phenomenon of autocatalysis in decomposition of energetic chemicals. Thermochim Acta. 2002;392:371–83.
Product information (2012) TAM III thermostat. www.thermometric.com.
Tseng JM, Liu MY, Chen SL, Hwang WT, Gupta JP, Shu CM. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III test. J Therm Anal Calorim. 2009;96:789–93.
Calorimetric principles (2008) TAM III training course, Taiwan, ROC
Calibration (2007) TAM III training course, Taiwan, ROC
Tseng JM, Chang YY, Su TS, Shu CM. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J Hazard Mater. 2007;142:765–70.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Liu SH, Lin CP, Shu CM. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J Therm Anal Calorim. 2011;106:165–72.
Gold V, Loening K, Sehmi P. Compendium of chemical terminology. IUPAC recommendations. Oxford: Blackwell Scientific Publications; 1987.
Lee JY, Choi HK, Shim MJ, Kim SW. Kinetic studies of an epoxy cure reaction by isothermal DSC analysis. Thermochim Acta. 2000;343:111–7.
Tsai LC, Chen JW, Hou HY, Liu SH, Shu CM. Exothermic behaviors in decomposition of three solid organic peroxides by DSC and VSP2. J Therm Anal Calorim. 2012;109:1303–9.
Acknowledgments
The authors are indebted to the donors of the National Science Council (NSC) in Taiwan under the contract number NSC-99-2221-E-224-029-MY3 for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, TS., Liu, SH., Qian, XM. et al. Isothermal hazards evaluation of benzoyl peroxide mixed with benzoic acid via TAM III test. J Therm Anal Calorim 113, 1625–1631 (2013). https://doi.org/10.1007/s10973-013-3020-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3020-8