Abstract
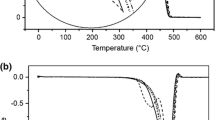
The thermal degradation of polypropylene-containing pro-oxidants was studied by determining the oxidation induction time (OIT) and by assessing the activation energy (E a) estimated from thermogravimetric analysis. Polypropylene (PP) was prepared with different concentrations of two pro-oxidants, polyacetal (POM) and d2w®. The pro-oxidants accelerated the oxidation process of oxidation of PP in the presence of oxygen; however, there is little change in the values of the OIT in compositions with different concentrations of d2w®. For PP/POM blends, the volatile low molecular mass compounds, primarily from POM-derived formaldehyde, accounted for the decrease in E a with the increasing POM concentration.






Similar content being viewed by others
References
AlMaaded MA, Madi NK, Hodzic A, Soutis C. Influence of additives on recycled polymer blends. J Therm Anal Calorim. 2013. doi:10.1007/s10973-013-3224-y.
Faxue Li F, Luo S, Zhang J, Yu J. Temperature dependences of solid structure and properties of biodegradable poly(butylene succinate-co-terephthalate) (PBST) copolyester. J Therm Anal Calorim. 2013;113:915–21.
Tzoganakis C, Vlachopoulos J, Hamielec AE. Production of controlled-rheology polypropylene resins by peroxide promoted degradation during extrusion. Polym Eng Sci. 1988;28:170–80.
Ryu SH, Gogos CG, Xanthos M. Parameters affecting process efficiency of peroxide-initiated controlled degradation of polypropylene. Adv Polym Technol. 1991;11:121–31.
Rocha MCG, Coutinho FMB, Balke S. Índice de fluidez: uma variável de controle de processos de degradação controlada de polipropileno por extrusão reativa. Polímeros Ciência e Tecnologia. 1994;3:16–22.
Kim BK. Reactive extrusion of polyolefins and their blends. Korea Polym J. 1996;4:215–26.
Berzin F, Vergnes B, Dufossé P, Delamare L. Modeling of peroxide initiated controlled degradation of polypropylene in a twin screw extruder. Polym Eng Sci. 2000;40:344–56.
Fontanella S, Bonhomme S, Brusson J-M, Pitteri S, Samuel G, Pichon G, Lacoste J, Fromageot D, Lemaire J, Delort A-M. Comparison of biodegradability of various polypropylene films containing pro-oxidant additives based on Mn, Mn/Fe or Co. Polym Degrad Stab. 2013;98:875–84.
Lucas N, Bienaime C, Belloy C, Queneudec M, Silvestre F, Saucedo J-E. Polymer biodegradation: mechanisms and estimation techniques. Chemosphere. 2008;73:429–42.
Position. Paper. July 2012 European Bioplastics, comments on the study. “A life cycle assessment of oxobiodegradabe, compostable and conventional bags” (May 2012, Intertek). DIALOG. http://www.google.com.br/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CCsQFjAA&url=http%3A%2F%2Fen.european-bioplastics.org%2Fwp-content%2Fuploads%2F2012%2F07%2FPositionspaper_LCA_160712.pdf&ei=ZANYUrK9DIWK9QSmnoG4Cw&usg=AFQjCNFGgdl3B6DDlGeKTUerJXsZW8SlUw&bvm=bv.53899372,d.eWU&cad=rja (2012). Accessed 15 Jan 2013.
Kong Hu Y, Song L, Tang Y. Kinetics of thermo-oxidative degradation of polypropylene/aluminum trihydroxide/organo Fe-montmorillonite nanocomposites Qinghong. J Therm Anal Calorim. 2011;104:1145–51.
Cottin H, Gazeau M-C, Doussin J-F, Raulin F. An experimental study of the photodegradation of polyoxymethylene. J Photochem Photobiol A. 2000;135:53–64.
Kannan M, Bhagawan SS, Thomas S, Joseph K. Thermogravimetric analysis and differential scanning calorimetric studies on nanoclay-filled TPU/PP blends. J Therm Anal Calorim. 2013;112:1231–44.
Zabihi O, Khodabandeh A. Understanding of thermal/thermo-oxidative degradation kinetics of polythiophene nanoparticles. J Therm Anal Calorim. 2013;112:1507–13.
American Society for Testing and Materials. Standard test method for decomposition kinetics by thermogravimetry, ASTM E1641-07. West Conshohocken: ASTM International. 2007.
Bouhelal SM, Cagiao ME, Bartolotta A, Marco GD, Benachour D, Calleja FJB. On polyethylene chain generation through chemical crosslinking of isotactic polypropylene. J Appl Polym Sci. 2010;116:394–403.
Gröning M, Hakkarainen M. Headspace solid-phase microextraction with gas chromatography/mass spectrometry reveals a correlation between the degradation product pattern and changes in the mechanical properties during the thermooxidation of in-plant recycled polyamide 6,6. J Appl Polym Sci. 2002;86:3396–407.
Wang X, Yu W, Nie Q, Guo Y, Du J. A real-time study on the evolution of the degradation of polypropylene during mixing process. J Appl Polym Sci. 2011;121:1220–43.
Gröning M, Hakkarainen G, Albertsson A-C. Quantitative determination of volatiles in polymers and quality control of recycled materials by static headspace techniques. In: Albertsson A-C, Hakkarainen M, editors. Chromatography for sustainable polymeric materials renewable, degradable and recyclable. Berlin: Springer; 2008. p. 51–84.
Eriksson P, Reitbergerb T, Stenberga B. Gas-phase contribution to the spreading of oxidation in polypropylene as studied by imaging hemiluminescence. Polym Degrad Stab. 2002;78:183–9.
Duan Y, Li H, Ye L, Liu X. Study on the thermal degradation of polyoxymethylene by thermogravimetry–fourier transform infrared spectroscopy (TG–FTIR). J Appl Polym Sci. 2006;99:3085–92.
Callister WD Jr. Ciência e Engenharia de Materiais: Uma Introdução. 2nd ed. Rio de Janeiro: LTC; 2006. p. 125.
Eriksson P, Reitberger T, Ahlblad G, Stenberg B. Oxidation fronts in polypropylene as studied by imaging chemiluminescence. Polym Degrad Stab. 2001;73:177.
Celina M, Clough RL, Jones GL. Initiation of polymer degradation via transfer of infectious species. Polym Degrad Stab. 2006;91:1036–44.
Acknowledgements
The authors thank UFABC and CAPES for financial support and scholarships. Derval thank FAPESP 2012/13445-8 the process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho, C.L., Rosa, D.S. Thermal oxidative degradation of polypropylene-containing pro-oxidants. J Therm Anal Calorim 115, 1627–1632 (2014). https://doi.org/10.1007/s10973-013-3490-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3490-8