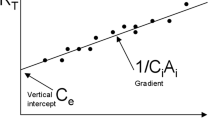
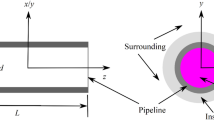
Abstract
The history of the term inertia is mentioned, and the effect of the heat inertia phenomenon is found in equations derived by Newton and Tian as well as in works of Vold and Borchard and Daniels. The mathematical correction of heat inertia consequences can be portrayed using the both differential and/or integral forms. It has been confirmed by the effective rectification applied to DTA (and heat-flux DSC) responses reflecting well the need of heat inertia corrections as to attain the original shape of inserted rectangular heat pulse.


Similar content being viewed by others
References
Kepler J. Epitome AstronomiaeCopernicanae: Usitata forma Quaestionum & Responsionum conscripta, inque VII. Librosdigesta, (1618–21; Epitome of Copernican Astronomy) Schönwetterus, 1635.
Newton I. Philosophiæ Naturalis Principia Mathematica (Mathematical Principles of Natural Philosophy) Londini, jussi Societatus Regiae ac typis Josephi Streater; prostatapud plures bibliopolas, 1687.
Newton I. Scale graduum caloris. Calorum descriptiones & signa. Philosophical Trans. 1701;22:824–29.
Tian A. Recherches sue la calorimétrie. Généralisation de la méthode de compensation électrique: Microcalorimétrie. J de Chimie-Physiq. 1933;30:665–708.
Vold MJ. Differential thermal analysis. Anal Chem. 1949;21:683–8.
Nevřiva M, Holba P, Šesták J. Utilization of DTA for the determination of transformation heats. Silikáty (Prague). 1976;29:33–9 (in Czech).
Šesták J, Holba P, Lombardi G. Quantitative evaluation of thermal effects: theory and practice. Annali di Chimica (Roma). 1977;67:73–87.
Holba P. On the applicability of isothermal kinetic equations for non-isothermal investigations of heterogeneous processes, thermal analysis . In: Buzas I, editor. Proc. 4th ICTA, Budapest 1974. AkadémiaiKiadó, Budapest 1975, Vol. 1, pp. 33–46, ISBN 963 05 0557 6.
Šesták J, Differential thermal analysis. In: Thermophysical properties of solids: theoretical thermal analysis. Chap. 12, Elsevier: Amsterdam 1984 (ISBN 0 444 99653 2), Czech origin by Academia, Praha 1984 and Russian translation by Mir, Moscow 1988.
Xue Y, Cracknell AP. Advanced thermal inertia modeling. Int J Remote Sens. 1995;16:431–46.
Šesták J. Is the original Kissinger equation obsolete today: Not obsolete the entire non-isothermal kinetics? J Therm Anal Calorim. 2014;117:3–7.
Faktor MM, Hanks R. Quantitative application of dynamic differential calorimetry. Part 1.—Theoretical and experimental evaluation. Trans Faraday Soc. 1967;63:1122–9.
Holba P, Nevřiva M. Description of thermoanalytical curves and the analysis of DTA peak by means of computer technique. Silikáty (Prague). 1977;21:19–23 (in Czech).
Holba P, Nevřiva M, Šesták J. Analysis of DTA curve and related calculation of kinetic data using computer technique. Thermochim Acta. 1978;23:223–31.
Chen R, Kirsh Y. Analysis of thermally stimulated processes. Oxford: Pergamum Press; 1981. p. 109–10.
Boerio-Goates J, Callen JE. Differential thermal methods. In: Rossiter BW, Beatzold RC, editors. Determination of thermodynamic properties, vol. 8. New York: Wiley; 1992. p. 621–718.
Borchardt HJ, Daniels F. The application of DTA to the study of reaction kinetics. J Am Chem Soc. 1957;79:41–6.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Holba P, Šesták J. Imperfections of Kissinger evaluation method and crystallization kinetics. Glass Physics and Chemistry. 2014; 40: 486–495. (ISSN 1087-6596; doi:10.1134/S1087659614050058) and on Russian: Fizika I Khimiya Stekla, 2014; 40:645–57.
Šesták J, Holba P, Živkovič Ž. Doubts on Kissinger´s method of kinetic evaluation based on several conceptual models showing the difference between the maximum of reaction rate and the extreme of a DTA. J Min Metall Sect B Metall. 2014;50:77–81. doi:10.2298/JMMB130902006S.
Šesták J. Rationale and fallacy of thermoanalytical kinetic patterns: how we model subject matter. J Therm Anal Calor. 2012;110:5–16.
Piloyan GO, Ryabchikov IO, Novikova SO. Determination of activation energies of chemical reactions by DTA. Nature. 1966;3067:1229.
O´Neill MJ. Analysis of the temperature controlled calorimeter. Anal Chem. 1964;36:1238–46.
Danley RL. (TA Instruments, Inc.): Power compensation differential scanning calorimeter EP 1136803 A1 (2001) http://www.google.com/patents/EP1136803A1.
Kaisersberger E, Moukhina E. Temperature dependence of the time constants for deconvolution of heat flow curves. Thermochim Acta. 2009;492:101–9.
Šesták J, Holba P. Heat inertia and temperature gradient in the treatment of DTA peaks: existing on every occasion of real measurements but until now omitted. J Therm Anal Calorim. 2013;113:1633–43.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Chao-Rui L, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of kinetic analysis Part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Holba P, Šesták J, Sedmidubský D, Heat transfer and phase transition at DTA experiments. In: Šesták J, Šimon P, editors. Thermal analysis of micro-, nano- and non-crystalline materials. 2013. Springer: Berlin, Chap. 5, p. 99–134 (ISBN 978 90 481 3149 5).
Acknowledgements
The results were developed within the CENTEM project, Reg. No. CZ.1.05/2.1.00/03.0088 that is co-funded from the ERDF as a part of the MEYS—Ministry of Education, Youth and Sports OP RDI Program and, in the follow-up sustainability stage supported through the CENTEM PLUS (LO 1402) by financial of above MEYS under the “National Sustainability program I”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holba, P., Šesták, J. Heat inertia and its role in thermal analysis. J Therm Anal Calorim 121, 303–307 (2015). https://doi.org/10.1007/s10973-015-4486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4486-3