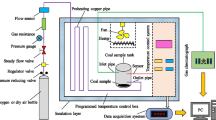
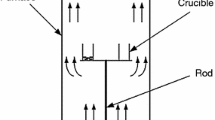
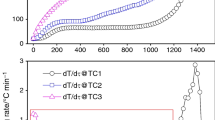
Abstract
The diffusion of oxygen (O2) plays an important role in the heterogeneous oxidation of coal and biomass, but is inadequately understood. This work aims to study the influence of intra-, inter-particle and external O2 diffusions on the high-temperature heterogeneous oxidation using the TG-FTIR technique and two bituminous coals as example. Results show that coal sample of higher reactivity and smaller pore surface area is more sensitive to the O2 diffusion. Specifically, increasing the size of particle, the reduced intra-particle (Knudsen) diffusion can reduce the conversion rate by 10–50%. While increasing the size of sample, the effective inter-particle diffusion shows a linear decrease. Comparatively, the influences of inter-particle and external diffusion in the TG scale (<5 mm) are weaker. For large TG samples (>10 mg) and low heating rates (2 K min−1), the influence of thermal diffusion is strong enough to cause a thermal leap for the oxidation. Kinetic analysis using nth-order model-fitting method predicts the apparent activation energy (E) decreases with increasing reactivity. However, both model-free and Kissinger’s methods show E increases with increasing reactivity, against the physical definition of E. This work may help understand the diffusion–kinetics interaction in the fuel conversion and smoldering fire of coal and biomass.











Similar content being viewed by others
References
Brown NJ, Bastien LA, Price PN. Transport properties for combustion modeling. Prog Energ Combust. 2011;37:565–82.
Kumar A, Mazumder S. Assessment of various diffusion models for the prediction of heterogeneous combustion in monolith tubes. Comput Chem Eng. 2008;32:1482–93.
Wang H, Dlugogorski BZ, Kennedy EM. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog Energ Combust. 2003;29:487–513.
Krishnaswamy S, Bhat S, Gunn RD. Low–temperature oxidation of coal 1. A single–particle reaction–diffusion model. Fuel. 1996;75:333–43.
Wang H, Dlugogorski B, Kennedy E. Theoretical analysis of reaction regimes in low–temperature oxidation of coal. Fuel. 1999;78:1073–81.
Huo W, Zhou Z, Wang F, Wang Y, Yu G. Experimental study of pore diffusion effect on char gasification with CO2 and steam. Fuel. 2014;131:59–65.
Carpenter DL, Sergeant GD. The initial stages of the oxidation of coal with molecular oxygen. III. Effect of particle size on rate of oxygen consumption. Fuel. 1966;45:311–27.
Akgun F, Arisoy A. Effect of particle size on the spontaneous heating of a coal stockpile. Combust Flame. 1994;99:137–46.
Bouwman R, Freriks IL. Low-temperature oxidation of a bituminous coal. Infrared spectroscopic study of samples from a coal pile. Fuel. 1980;59:315–22.
Gómez-Barea A, Ollero P, Fernández-Baco C. Diffusional effects in CO2 gasification experiments with single biomass char particles. 1. Experimental investigation. Energ Fuel. 2006;20:2202–10.
Gómez-Barea A, Ollero P, Leckner B. Mass transport effects during measurements of gas–solid reaction kinetics in a fluidised bed. Chem Eng Sci. 2007;62:1477–93.
Huo W, Zhou Z, Wang F, Yu G. Mechanism analysis and experimental verification of pore diffusion on coke and coal char gasification with CO2. Chem Eng Sci. 2014;244:227–33.
Mani T, Mahinpey N, Murugan P. Reaction kinetics and mass transfer studies of biomass char gasification with CO2. Chem Eng Sci. 2011;66:36–41.
Ollero P, Serrera A, Arjona R, Alcantarilla S. Diffusional effects in TGA gasification experiments for kinetic determination. Fuel. 2002;81:1989–2000.
Vincent SS, Mahinpey N, Aqsha A. Mass transfer studies during CO2 gasification of torrefied and pyrolyzed chars. Energy. 2014;67:319–27.
Huang X, Rein G. Thermochemical conversion of biomass in smouldering combustion across scales: the roles of heterogeneous kinetics, oxygen and transport phenomena. Biosource Technol. 2016;207:409–21.
Huang X, Rein G. Smouldering combustion of peat in wildfires: Inverse modelling of the drying and the thermal and oxidative decomposition kinetics. Combust Flame. 2014;161:1633–44.
Wu D, Schmidt M, Huang X, Verplaetsen F. Self-ignition and smouldering of coal dust accumulations in O2/N2 and O2/CO2 atmospheres. Proc Combust Inst. 2017;36:3195–202.
Hull A, Lanthier JL, Agarwal PK. The role of the diffusion of oxygen in the ignition of a coal stockpile in confined storage. Fuel. 1997;76:975–83.
Hull AS, Lanthier JL, Chen Z, Agarwal PK. The role of the diffusion of oxygen and radiation on the spontaneous combustibility of a coal pile in confined storage. Combust Flame. 1997;110:479–93.
Zhang Y, Wu J, Chang L, Wang J, Li Z. Changes in the reaction regime during low–temperature oxidation of coal in confined spaces. J Loss Prevent Proc. 2013;26:1221–9.
Everson RC, Neomagus HW, Kaitano R. The random pore model with intraparticle diffusion for the description of combustion of char particles derived from minera and inertinite rich coal. Fuel. 2011;90:2347–52.
Gómez-Barea A, Ollero P, Arjona R. Reaction–diffusion model of TGA gasification experiments for estimating diffusional effects. Fuel. 2005;84:1695–704.
Anthony EJ. Fluidized bed combustion of alternative solid fuels; status, successes and problems of the technology. Prog Energ Combust. 1995;21:239–68.
Liu B, Yang X, Song W, Lin W. Process simulation development of coal combustion in a circulating fluidized bed combustor based on aspen plus. Energ Fuel. 2011;25:1721–30.
Murakami T, Suzuki Y, Durrani AK. New Approach to understanding NO emission during bubbling fluidized bed coal combustion: separation of NO formation and reduction processes in the bed. Energy Fuel. 2010;23:1950–5.
Glazer MP, Khan NA, de Jong W, Spliethoff H, Schürmann H, Monkhouse P. Alkali metals in circulating fluidized bed combustion of biomass and coal: measurements and chemical equilibrium analysis. Energy Fuel. 2005;19:1889–97.
Belo LP, Spörl R, Shah KV, Elliott LK, Stanger RJ, Maier J, Wall TF. Sulfur capture by fly ash in air and oxy–fuel pulverized fuel combustion. Energ Fuel. 2014;28:5472–9.
Nunes KGP, Osório E, Marcílio NR. Kinetics of the oxy–fuel combustion of high-ash-content coal from the Candiota mine. Rio Grande do Sul. Energ Fuel. 2016;30:1958–64.
Buhre BJP, Elliott LK, Sheng CD, Gupta RP, Wall TF. Oxy-fuel combustion technology for coal-fired power generation. Prog Energ Combust. 2005;31:283–307.
Bhutto AW, Bazmi AA, Zahedi G. Underground coal gasification: From fundamentals to applications. Prog Energ Combust. 2013;39:189–214.
Peng P, Barse K, Nasah J. Competitiveness and cost sensitivity study of underground coal gasification combined cycle using lignite. Energ Fuel. 2016;30:2111–8.
And GP, Sahajwalla V. A Mathematical model for the chemical reaction of a semi-infinite block of coal in underground coal gasification. Energ Fuel. 2005;19:1679–92.
Vyazovkin S, Chrissafis K, Di Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Ramachandran P. Analytical prediction of conversion-time behaviour of gas-solid noncatalytic reactions. Chem Eng Sci. 1983;38:1385–90.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Carras JN, Young BC. Self-heating of coal and related materials: models, application and test methods. Prog Energ Combust. 1994;20:1–15.
Kim RG, Jeon CH. Intrinsic reaction kinetics of coal char combustion by direct measurement of ignition temperature. Appl Therm Eng. 2014;63:565–76.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Altun NE, Kok MV, Hicyilmaz C. Effect of particle size and heating rate on the combustion of silopi asphaltite. Energ Fuel. 2002;16:785–90.
Garciamaraver A, Perezjimenez JA, Serranobernardo F, Zamorano M. Determination and comparison of combustion kinetics parameters of agricultural biomass from olive trees. Renew Energ. 2015;83:897–904.
Shen DK, Gu S, Jin B, Fang MX. Thermal degradation mechanisms of wood under inert and oxidative environments using DAEM methods. Bioresour Technol. 2011;102:2047–52.
Wu D, Huang X, Norman F, Verplaetsen F, Berghmans J, Van den Bulck E. Experimental investigation on the self-ignition behaviour of coal dust accumulations in oxy-fuel combustion system. Fuel. 2015;160:245–54.
Kök MV, Özbas E, Karacan O, Hicyilmaz C. Effect of particle size on coal pyrolysis. J Anal Appl Pyrol. 1998;45:103–10.
Güldoğan Y, Durusoy T, Bozdemir T. Effects of heating rate and particle size on pyrolysis kinetics of gediz lignite. Energ Source. 2002;24:753–60.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Li KY, Huang X, Fleischmann C, Rein G, Ji J. Pyrolysis of medium-density fiberboard: optimized search for kinetics scheme and parameters via a genetic algorithm driven by Kissinger’s method. Energ Fuel. 2014;28:6130–9.
Ertunc G, Kok MV. Determination of kinetic parameters of different origin coals using software. J Therm Anal Calorim. 2015;119:1–7.
Kok MV. Simultaneous thermogravimetry–calorimetry study on the combustion of coal samples: effect of heating rate. Energ Convers Manage. 2012;53:40–4.
Altun NE, Hicyilmaz C, Kök MV. Effect of particle size and heating rate on the pyrolysis of Silopi asphaltite. J Anal Appl Pyrol. 2003;67:369–79.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.: 51506081), Natural Science Foundation of Jiangsu Province (No.: BK20150954) and the Open Fund of the State Key Laboratory of Fire Science (SKLFS) Program (HZ2015-KF09). Authors appreciate Mr. Wenyu Qi and Mr. Yinshui Long from the Henan Energy and Chemical Industry Group Co., LTD for collecting coal samples. Thanks to anonymous reviewers’ and Editor-in-Chief’s valuable suggestions for improving the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Z., Huang, X., Luo, M. et al. Experimental study on the diffusion–kinetics interaction in heterogeneous reaction of coal. J Therm Anal Calorim 129, 1625–1637 (2017). https://doi.org/10.1007/s10973-017-6386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6386-1