Abstract
This work reports on the thermal stability and flammability of a novel class of rigid polyurethane foams chemically modified by functionalized 1,2-propanediolisobutyl POSS (PHI-POSS) as a pendant group and octa (3-hydroxy-3-methylbutyldimethylsiloxy) POSS (OCTA-POSS) as a chemical cross-link. The foamed hybrid materials were prepared in a three-step process using a sorbitol-based polyether polyol, polymeric 4,4′-diphenylmethane diisocyanate and dimethyl propane phosphonate as a flame retardant. The addition of the POSS modifier influences the PU cellular structure as evidenced by a change in anisotropy index of the cross section parallel to the growth direction. Based on thermogravimetric data and flammability results, one can suggest that there is char formation at the surface and the formed layer acts as an insulating barrier limiting heat and mass transfer with flame retardant and thus leading to decreased heat release rate, especially for systems containing OCTA-POSS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyurethanes (PUs) are a class of polymers with broad range of properties and applications. Their many uses include rigid foams as insulation in walls and roofs, flexible foams in upholstered furniture and thermoplastic polyurethanes, e.g., adhesives and coatings, as well as biomedical materials [1–4]. Polyurethanes have increasingly been used during the past thirty years in a variety of applications due to their ease of synthesis, low cost, energy efficiency and potential environmental friendliness. The mechanical, thermal, insulating, etc., properties of PU foams can be tailored by using appropriate polyisocyanates and polyols as well as by combining them with various nanofillers [3, 5–12].
Unmodified PU foams are highly cellular materials that are easily ignitable and highly flammable, which is a factor that limits their broader use [13] and causes a demand for fire-retarding additives. Apart from traditional halogenated and non-halogenated fire retardants, new alternatives are being tested, e.g., polyhedral oligomeric silsesquioxanes (POSS). They are unique nanobuilding blocks that can be used to obtain a wide variety of hybrid materials with a range specific properties [14–16]. POSS consist of a siliceous cage-like core with Si atoms on the vertices and O atoms on the edges. Organic ligands are attached on the Si atoms which allow the cage-like cores to be directly bound to the polymer chains, via covalent linkage [17, 18]. These siliceous cages have sizes of 1–3 nm and can be considered as the smallest possible particles of silica. Reactive POSS moieties can be bonded with macromolecules during the polymerization reaction or by a reactive blending approach [19]. POSS moieties were found to strongly influence mechanical, thermal and barrier properties of polymer matrices. Special attention was focused on the investigations of flame retardancy effects of POSS—for instance, Lichtenhan et al. have investigated poly(ether-block-amide)s reinforced by POSS and they found that the peak of the heat release rate (PHRR) measured by cone calorimetry was decreased by 77% as compared to the pristine polymer [20]. Along this line of interest, Fina et al. [21] reported on the applications of dimeric and oligomeric Al- and Zn-isobutyl silsesquioxane (POSS) in polypropylene (PP)—the incorporation of Al-POSS in PP caused a decrease in combustion as compared to PP, evidenced by a decrease of the heat release rate as well as reduction in CO and CO2 production rates. Bourbigot et al. in a search to replace traditional halogenated fire retardants with non-halogenated, nanostructured alternatives, investigated by mass loss calorimetry the reaction to fire of thermoplastic polyurethane (TPU) containing poly(vinylsilsesquioxane). Large reduction of peak of heat release rate (PHRR) in comparison with pristine TPU was observed, and the protection mechanism was found to occur via an intumescent mechanism [22]. Recently, an attempt to evaluate the thermal stability and flame retardancy of POSS-containing polymer nanocomposites, including determination of structure–property relationships, was made by Qian and co-workers [23]. Generally, metal-containing POSS show good catalytic charring ability, whereby vinyl- and phenyl-containing POSS promote the strength of char. All POSS materials may be considered as ceramic precursors that are able to protective surface layer.
Polyurethane foams with chemically built-in POSS cages are a new class of foamed materials that has not been yet reported in the literature, apart from our just performed studies [24, 25].
Hence, in this work we report for the first time on the thermal stability and flammability of novel class of polyurethane foams chemically modified by functionalized 1,2-propanediolisobutyl POSS (PHI-POSS) as a pendant group and octa (3-hydroxy-3-methylbutyldimethylsiloxy) POSS (OCTA-POSS) as a chemical cross-link, flame retarded by dimethyl propane phosphonate (DMPP).
Experimental
Raw materials
Polyurethane foams (PUF) were synthesized using (i) Rokopol RF551, a general purpose, sorbitol-based polyether polyol recommended for the production of rigid polyurethane foams and acquired from PCC Rokita S.A. and (ii) polymeric 4,4′-diphenylmethane diisocyanate (polymeric MDI) acquired from Minova Ekochem under the trade name Ekopur B. As auxiliary agents silicone SR-321 from Momentive Performance Materials Inc. (a non-hydrolyzable silicone surfactant for rigid foam formulations, such as those having very low reactivities) and Polycat® 9 catalyst from Air Products, a low odor tertiary amine that provides a balanced promotion of the urethane and urea reactions in rigid foam applications, were applied. Levagard® DMPP (dimethyl propane phosphonate) was used as a halogen free flame retardant with a very high phosphorus content.
The POSS: 1,2-propanediolisobutyl POSS (PHI-POSS) and octa (3-hydroxy-3-methylbutyldimethylsiloxy) POSS (OCTA-POSS) were purchased from Hybrid Plastics.
Synthesis
For the synthesis of polyurethane foams, a three-step process was applied—at the first stage, polyol (polyether RF-551) was stirred with POSS (1,2-propanediolisobutyl POSS and octa (3-hydroxy-3-methylbutyldimethylsiloxy) POSS) dissolved in THF using an ultrasonic homogenizer. Then, catalyst (Polycat 9), water, surfactant (SR-321) and flame retartant (Levagard® DMPP) were added in order to prepare the polyol premix (component A). In the next step, n-pentane as a physical blowing agent was added to component A. In the third step, component B (polymeric 4,4′-diphenylmethane diisocyanate (PMDI)) was added to component A and the mixture was stirred for 15 s with an overhead stirrer. Formulation of the obtained PU-POSS foams is given in Table 1.
Finally, the prepared mixtures were dropped into a Teflon open mold. All the experiments were performed at ambient temperature of ca. 20 °C.
Methods
Thermal conductivity
Measurements of thermal conductivity were carried out by means of a Laser Comp Heat Flow Instrument Fox 200 designed according to ISO 8301. Method of measuring coefficient of thermal conductivity (λ) determines the amount of heat flow through a material per unit of time during a steady flow of heat, at constant temperature difference on opposite sides of the test sample material. A test piece of dimensions 200 × 200 × 25 mm3 is placed in a chamber between two isothermal plates, which the lower plate is movable. The method of measurement is based on one-way flow of heat, which describes the Fourier equation.
The temperature gradient is expressed by a temperature difference of hot and cold plates relative to the thickness of the sample. Measurements were performed after 24 h from the receipt of foam at an average temperature of 10 °C, setting the appropriate cold temperature of 0–20 °C hot plate. The samples were placed in a dry room at room temperature. After period of 7 days, the test was repeated.
Mechanical properties
Measurements of compressive strength were carried out according to the standard ISO 844. For the obtained foam, after cutting skins and sides samples were cut in the shape of cubes with an edge length 25 mm. Then, the samples were subjected to 10% compressive deformation by means of testing machine Zwick Allround 7005 and checked for compressive strength in the direction parallel and perpendicular to the direction of foam rise.
The contents of closed cell
The method of measuring the content of closed cells is suitable for determining the percentage by volume of closed cells in rigid plastics porous according to ISO 4590. Samples with dimensions of 20 × 20 × 100 mm3, without deformation, are placed in a sealed chamber of apparatus. The principle of method is that the volume of gas ejected from the sample when it is placed in the chamber shall be determined based on changes in the pressure of the remaining air surrounding the sample, where it is increased by a known volume (expansion into a known volume).
Analysis of the cellular structure
The analysis of the cellular structure was conducted using Aphelion™ software. The program can set parameters such as surface area of the cells, cell number and the anisotropy index of the materials obtained.
Attenuated total reflectance-Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy measurements were performed on a Nicolet iS5 spectrometer (Thermo Scientific) equipped with diamond crystal attenuated total reflectance unit. Spectra were recorded with a resolution of 4 cm−1 from 4000 to 400 cm−1 with an average of 16 scans.
Analysis of the energy-dispersive spectroscopy (EDS)
Scanning electron microscopy (SEM) micrographs were recorded with a JEOL In Touch Scope JSM-6010LV scanning electron microscope (Tokyo, Japan) with energy-dispersive X-ray analysis capabilities, operated at 10-kV accelerating voltage.
Thermogravimetry—TG
Thermogravimetric analysis was performed using a Netzsch TG 209 thermal analyzer to investigate the thermal stability of the obtained PU/POSS/DMPP foams. The samples (about 5 mg) were heated in an open α-Al2O3 pan from 25 up to 600 °C at a heating rate of 10 °C · min−1 under air atmosphere (air flow 30 cm3 · min−1).
Limiting oxygen index—LOI
The oxygen index is defined as the minimum volume concentration of oxygen in a mixture of oxygen and nitrogen introduced at 23 ± 2 °C that will just support combustion of a material under specified test conditions, according to ISO 4589-2.
Microcalorimetry PCFC
The pyrolysis combustion flow calorimetry (PCFC) technique, developed by Fire Testing Technology Ltd. (UK), uses traditional oxygen depletion calorimetry. The sample is first heated at a constant rate of temperature rise of 1 deg s−1 in a pyrolyzer. The thermal decomposition products are swept from the pyrolyzer by an inert gas. After pyrolysis, the gas stream is mixed with oxygen and enters a combustor at 900 °C, where products after decomposition are completely oxidized. Oxygen concentrations and flow rates of the combustion gases are used to determine the oxygen depletion involved in the combustion process and the heat release, as well as the heat release capacity.
Results and discussion
Analysis of the cellular structure
Analysis of the cellular structures of obtained material was performed on cross section both parallel and perpendicular to the growth direction of the material. Since the polyurethane foams are anisotropic materials, the cellular structure needs to be analyzed in two directions relative to the growth direction of the material. PHI-POSS additive to the rigid polyurethane foam systems caused an increase in average cross-sectional area of the cells and a slight decrease in the number of cells per 1 mm2 in cross section parallel in comparison with the reference material (Table 2).
The anisotropy index in the cross section increased to 10% of the PHI-POSS, while for 15% of the added modifier reached a value lower than the reference material. However, in the perpendicular cross section initial increase was observed and then decrease in the number of cells with the highest modifier content that showed the highest mean cross-sectional surface (Table 2).
For foams modified with OCTA-POSS, no apparent change with increasing modifier introduced into the foam system was observed. The anisotropy index was changed only in the parallel direction, since it has reduced for the foams containing 10 and 15 mass% of OCTA-POSS (Table 3).
Content of closed cells for foams containing PHI-POSS is comparable to the reference material. In contrast, in the system comprising OCTA-POSS reduction in the quantity of closed cells, especially for the modifier content of 10 mass%, was found (Fig. 1).
The addition of the modifier influences the cellular structure of the obtained materials as evidenced by changes in anisotropy index of the cross section parallel to the growth direction of PU foams.
ATR-Fourier transform infrared spectroscopy
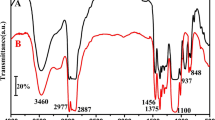
Figure 2 shows the spectra of the reference foam (neat PUF) and the hybrid foams containing 5, 10, 15 mass%. PHI-POSS and OCTA-POSS. A few characteristic vibration bands may be observed in the range 1750–1500 cm−1 (stretching of C = O group and C = C in aromatic ring), 1300–1200 cm−1 (stretching of C–O in alcohols functional groups of POSS) which are capable of reacting with isocyanate moieties. Moreover, there are characteristic asymmetric stretching vibrations of C–O–C group in the range 1200–1000 cm−1, whereby characteristic bands in the range 920–730 cm−1 arise from vibrations of silicon-containing groups. No peaks originating from the unreacted isocyanate component were observed in the characteristic spectral range of 2273–2240 cm−1 (Fig. 2).
Analysis of SEM pictures
In order to determine the effect of the additives on the microstructure of rigid polyurethane foams, scanning electron microscopy with EDS microanalysis was employed.
For systems containing PHI-POSS, no major changes resulting from the amount of introduced POSS were observed. POSS moieties show very good distribution on the surface of the foam, the same as for flame retardant applied (Fig. 3).
In the case of compositions containing OCTA-POSS, fine distribution of silicon can be seen; however, at POSS level of 15 mass% agglomeration of silsesquioxane takes place, as evidenced in Fig. 4f. Distribution of phosphorus present in DMPP is uniform on the surface of polyurethane foam (Fig. 4).
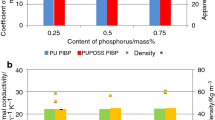
Thermal conductivity studies
Insulating properties of PU foams containing POSS were measured after 24 h. The measurement was repeated after seven days in order to check the changes in thermal conductivity. The largest increase in the coefficient of thermal conductivity was found for foam containing the largest amount of silsesquioxane (Fig. 5). Repeated testing after seven days showed an increase in this parameter, which is caused by the diffusion of a physical foaming agent, whose place is taken by the air affecting thus the heat-insulating properties.
Apparent density of foams containing 5 and 10 mass% of PHI-POSS was decreased due to the introduction of small amounts of tetrahydrofuran THF, which was used to dissolve the POSS in the polyol premix—Fig. 6. However, for silsesquioxane content of 15 mass% an increase in apparent density can be observed—this effect is caused by viscosity increase of the polyol premix in the presence of POSS nanoparticles.
For the foams modified by OCTA-POSS, there is an increase in the thermal conductivity for all the filler concentrations. After seven days, a slight increase in the coefficient of thermal conductivity, caused by the replacement of physical blowing agents, has been recorded. Foam containing 10 mass% of POSS modifier was characterized by a significant deterioration of thermal insulation properties resulting primarily from the low content of closed cells contributing to the increased exchange of physical blowing agents.
The apparent density of the foams containing OCTA-POSS is lower than density of the reference material which might be associated with the presence of additional physical foaming agent—THF that was used to facilitate the introduction of POSS into the polyol premix.
Mechanical properties studies
In order to determine the effect of the silsesquioxane modifiers on mechanical properties of anisotropic polyurethane foams, compressive strength was measured in the direction parallel (X) and perpendicular (Y, Z) to the growth direction.
PHI-POSS modified foams were characterized by reduced mechanical strength, regardless of the direction of the study which can be linked with a reduction in the apparent density of the materials obtained (Fig. 7). The smallest change in the compressive strength in the parallel direction was observed for hybrid composite containing 5 mass% of PHI-POSS. In the perpendicular direction, composite material containing 15 mass% of reactive filler was characterized by comparable mechanical properties as the reference material.
For foams containing OCTA-POSS, similar changes in the mechanical properties can be observed. The obtained composite materials are characterized by a reduced compressive strength regardless of the amount of modifier incorporated (Fig. 8). Changes in compressive strength are primarily associated with changes in the apparent density caused by introduction of the modifier together with a small amount of THF which increases the amount of a physical blowing agent and leads to plasticization of the polymer matrix.
Thermal stability investigations
The results of the thermogravimetric analysis of modified PU/POSS/DMPP foams under air atmosphere are shown in Fig. 9 and Table 4. The degradation process runs in three distinct stages with maximum of mass loss at ca. 316–322 °C and 537–546 °C.
First step of TG analysis could be attributed to the vaporization of DMPP which has a relatively low boiling point of 182 °C). No or small residue at 600 °C indicates mainly the gas phase activity of DMPP. However, the next two stages of degradation in the atmosphere of air PU materials involves the reaction of oxygen to form hydroperoxides which themselves are unstable and succumbing to decomposition to form more free radicals [26, 27].
POSS in combination with flame retardant may affect to stabilize the polyurethane material in the process of degradation that can influence the thermal stability [27].
Moreover, the addition of POSS to polyurethane foams with DMPP causes an increase in temperature at 50 mass% of mass loss and increases the amount of residue after the thermogravimetric analysis which is probably due to the formation of char containing polyphosphoric acids. This observation can indicate that POSS moieties stabilize the PU chains more effectively than the DMPP flame retardant alone.
Flammability studies
The silsesquioxanes addition caused a slight decrease in the LOI value compared to the reference material, regardless of the amount of the introduced modifier (Table 5).
Microcalorimetry results revealed that POSS moieties caused a change in the heat release rate (HRR) during burning of polyurethane foams. Interestingly, for PUF/DMPP/PHI-POSS system a decrease in HRR was observed which can be the result of a synergistic effect between the additives (Fig. 10a). In comparison with foams that contain DMPP flame retardant, only a substantial decrease in the peak HRR for all the compositions containing POSS was registered (Fig. 10b). Reduction of the heat release rate indicates that POSS species are supporting the flame retardant’s action probably thorough formation of a protective surface layer [27].
The microcalorimetry profiles of all the foams exhibit two decomposition peaks. The larger one occurs at the temperature range 300–400 °C and the weaker around 500 °C (Fig. 10); both steps correspond to the decomposition steps detected by TG under air atmosphere [27]. The incorporation of OCTA-POSS causes a systematic shift of both complex peaks to higher temperatures, indicating that the hybrid foams are more stable. One could explain this effect by the cross-linking abilities of OCTA-POSS leading to the formation of more dense network. The incorporation of pendant PHI-POSS moieties does not impose such a prominent effect due to its more loose arrangement in the PU matrix.
Taking into account thermogravimetric data [27] and flammability results, one can suggest that POSS moieties facilitate char formation at the surface and the formed layer acts as an insulating barrier limiting heat and mass transfer and thus leading to decreased heat release rate. It is in accordance with the results of previous investigations [23, 28, 29]. However, the fire behavior of foamed polyurethane materials is strongly influenced by their cellular structure that is modified in the presence of POSS nanocages.
Conclusions
Introduction to the rigid polyurethane foam of the two types of POSS and DMPP as a flame retardant influences the physical and mechanical properties of the materials obtained. This is caused by changes in apparent density and cell structure, which are closely related to the compressive strength. The materials obtained are characterized by small changes in the thermal insulation properties and a good distribution of applied additives. Only at the highest content of OCTA-POSS, a non-uniform dispersion of silsesquioxane in the polyurethane matrix can be observed.
In the PUF/POSS foamed materials, thermal stabilization effect due to the presence of POSS particles and DMPP is evidenced by a shift of the initial decomposition temperature. This may be explained as stabilization of the PU macrochains by bulky covalently linked substituents that reinforce the polymer against thermally induced motions leading to fragmentation and formation of macroradicals. It has been found that the POSS moieties stabilize the PU chains more effectively in the absence of reactive oxygen-containing species which are unlikely to interact with silica-based nanocages. Taking into account thermogravimetric data and flammability results, one can suggest that there is char formation at the surface and the formed layer acts as an insulating barrier limiting heat and mass transfer with flame retardant and thus leading to decreased heat release rate, especially for systems containing OCTA-POSS. One could explain this effect by the cross-linking abilities of OCTA-POSS leading to the formation of more dense network, whereby incorporation of pendant PHI-POSS moieties does not impose such a prominent effect due to its more loose arrangement in the PU matrix.
References
Randall D, Lee S, editors. The polyurethanes book. New York: Wiley; 2002.
Prisacariu C, editor. Polyurethane elastomers: from morphology to mechanical aspects. Wien: Springer; 2011.
Szycher M, editor. Szycher’s handbook of polyurethanes. New York: CRC Press; 2012.
Kurańska M, Prociak A, Cabulis U, Kirpiuks M. Water-blown polyurethane-polyisocyanurate foams based on bio-polyols with wood fibers. Polimery. 2015;60(11–12):705–12.
Engels H-W, Pirkl H-G, Albers R, Albach RW, Krause J, Hoffmann A, Casselmann H, Dormish J. Polyurethanes: versatile materials and sustainable problem solvers for today’s challenges. Angew Chem Int Ed. 2013;52:9422–41.
Paulino M, Teixeira-Dias F. On the use of polyurethane foam paddings to improve passive safety in crashworthiness applications. In: Polyurethane, chap. 15. Rijeka: InTech; 2012.
Ashida K, editor. Polyurethane and Related Foams: Chemistry and Technology. New York: CRC Press; 2006.
Salasinska K, Borucka M, Leszczyńska M, et al. Analysis of flammability and smoke emission of rigid polyurethane foams modified with nanoparticles and halogen-free fire retardants. J Therm Anal Calorim. 2017; doi:10.1007/s10973-017-6294-4.
Malewska E, Prociak A. The effect of nanosilica filler on the foaming process and properties of flexible polyurethane foams obtained with rapeseed oil-based polyol. Polimery. 2015;60(7–8):472–9.
Gao L, Zheng G, Zhou Y, Hu L, Feng G. Improved mechanical property, thermal performance, flame retardancy and fire behavior of lignin-based rigid polyurethane foam nanocomposite. J Therm Anal Calorim. 2015;120:1311–25.
Nikje MMA, Noruzian M, Moghaddam ST. Investigation of Fe3O4/AEAP supermagnetic nanoparticles on the morphological, thermal and magnetite behavior of polyurethane rigid foam nanocomposites. Polimery. 2015;60:26–32.
Ciecierska E, Jurczyk-Kowalska M, Bazarnik P, Kowalski M, Krauze S, Lewandowska M. The influence of carbon fillers on the thermal properties of polyurethane foam. J Therm Anal Calorim. 2016;123:283–91.
Singh H, Jain AK. Ignition, combustion toxicity and fire retardancy of polyurethane foams: a comprehensive review. J Appl Polym Sci. 2009;111:1115–43.
Rościszewski P, Kazimierczuk R, Sołtysiak J. Syntheses of silsesquioxanes with various organic substituents. Polimery. 2006;51(1):3–11.
Asuncion MZ, Laine RM. Silsesquioxane barrier materials. Macromolecules. 2007;40:555–62.
Blanco I, Bottino FA, Abate L. Influence of n-alkyl substituents on the thermal behaviour of Polyhedral Oligomeric Silsesquioxanes (POSSs) with different cage’s periphery. Thermochim Acta. 2016;623:50–7.
Pielichowski K, Njuguna J, Janowski B, Pielichowski J. Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. Adv Polym Sci. 2006;201:225–96.
Janowski B, Pielichowski K. Nano-hybrid polymers containing polyhedral oligosilsesquioxanes (POSS). Polimery. 2008;53(2):87–92.
Madbouly SA, Otaigbe JU, Nanda AK, Wicks DA. Rheological behavior of POSS/Polyurethane-Urea nanocomposite films prepared by homogeneous solution polymerization in aqueous dispersions. Macromolecules. 2007;40(14):4982–91.
Lichtenhan JD, Gilman JW. Preceramic additives as fire retardants for plastics. US Patent 6.362.279. 2002.
Fina A, Abbenhuis HCL, Tabuani D, Camino G. Metal functionalized POSS as fire retardants in polypropylene. Polym Degrad Stab. 2006;91(10):2275–81.
Bourbigot S, Turf T, Bellayer S, Duquesne S. Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym Degrad Stab. 2009;94(8):1230–7.
Qian Y, Wei P, Zhao X, Jiang P, Yu H. Flame retardancy and thermal stability of polyhedral oligomeric silsesquioxane nanocomposites. Fire Mater. 2013;37(1):1–16.
Pielichowski K, Hebda E, Michałowski S, Pielichowski J, Jancia M, Ozimek J. The preparation method of hybrid polyurethane nanocomposite. Polish patent application. No.P.408051; 2014.
Hebda E, Ozimek J, Raftopoulos KN, Michałowski S, Pielichowski J, Jancia M, Pielichowski K. Synthesis and morphology of rigid polyurethane foams with POSS as pendant groups or chemical crosslinks. Polym Adv Technol. 2015;26:932–94.
Turi EA, editor. Thermal characterization of polymeric materials. New York: Academic Press; 1997.
Pagacz J, Hebda E, Michałowski S, Ozimek J, Sternik D, Pielichowski K. Polyurethane foams chemically reinforced with POSS—thermal degradation studies. Thermochim Acta. 2016;642:85–104.
Devaux E, Rochery M, Bourbigot S. Polyurethane/clay and polyurethane/POSS nanocomposites as flame retarded coating for polyester and cotton fabrics. Fire Mater. 2002;26(4–5):149–54.
Zhang W, Camino G, Yang R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: an overview of fire retardance. Prog Polym Sci. 2017;67:77–125.
Acknowledgements
This project was financed by the Polish National Science Centre under contract No. DEC-2011/02/A/ST8/00409.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Michałowski, S., Hebda, E. & Pielichowski, K. Thermal stability and flammability of polyurethane foams chemically reinforced with POSS. J Therm Anal Calorim 130, 155–163 (2017). https://doi.org/10.1007/s10973-017-6391-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6391-4