Abstract
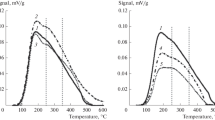
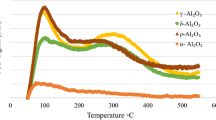
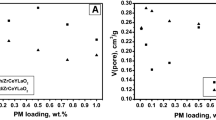
A series of Pd/Al2O3 catalysts were prepared by incipient wetness impregnation method. Palladium loading was varied in a range of 0.125–4.0 mass%. The catalytic performance of the samples was tested in model reaction of CO oxidation at oxygen excess. Catalysts were characterized by temperature-programmed reduction (TPR), electron paramagnetic resonance, and UV–visible spectroscopy. Thermal aging of chosen samples was performed at 1000 °C. The thermal aging behavior was studied using TPR, X-ray diffraction analysis, transmission electron microscopy, and X-ray photoelectron spectroscopy (XPS). It was shown that palladium stabilized in the form of dispersed surface Pd2+ species when Pd loading is 0.5 mass% and below. Samples with higher loading preferentially contain nanosized Pd particles. Agglomeration of Pd species during thermal aging was found to take place starting from Pd concentration of 1.0 mass%. In some cases, size of Pd particles exceeds 150 nm, which is in about 40 times higher comparing with the initial samples. According to XPS data, degree of Pd2+-alumina interaction in the aged samples is also increased.










Similar content being viewed by others
References
Hong UG, Hwang S, Seo JG, Yi J, Song IK. Hydrogenation of succinic acid to γ-butyrolactone over palladium catalyst supported on mesoporous alumina xerogel. Catal Lett. 2010;138:28–33.
Vishwanathan V, Jayasri V, Mahaboob Basha P. Vapor phase hydrogenation of o-chloronitrobenzene (o-CNB) over alumina supported palladium catalyst—a kinetic study. React Kinet Catal Lett. 2007;91:291–8.
Fukuyama T, Kippo T, Ryu I, Sagae T. Addition of allyl bromide to phenylacetylene catalyzed by palladium on alumina and its application to a continuous flow synthesis. Res Chem Intermed. 2009;35:1053.
Arora S, Kapoor P, Singla ML. Catalytic studies of palladium nanoparticles immobilized on alumina synthesized by a simple physical precipitation method. React Kinet Mech Catal. 2010;99:157–65.
Berenblyum AS, Podoplelova TA, Shamsiev RS, Katsman EA, Danyushevsky VY. On the mechanism of catalytic conversion of fatty acids into hydrocarbons in the presence of palladium catalysts on alumina. Pet Chem. 2011;51:336–41.
Gopinath R, Seshu Babu N, Vinod Kumar J, Lingaiah N, Sai Prasad PS. Influence of Pd precursor and method of preparation on hydrodechlorination activity of alumina supported palladium catalysts. Catal Lett. 2008;120:312–9.
Thomazeau C, Cseri T, Bisson L, Aguilhon J, Minh DP, Boissière C, Durupthy O, Sanchez C. Nano design of alumina supported monometallic catalysts: a promising way to improve the selective hydrogenation of poly-unsaturated hydrocarbons. Top Catal. 2012;55:690–9.
Cizmeci M, Musavi A, Tekin A, Kayahan M. Comparison of two palladium catalysts on different supports during hydrogenation. J Am Oil Chem Soc. 2006;83:1063–8.
Karpiński Z, d’Itri JL. Hydrodechlorination of 1,1-dichlorotetrafluoroethane on supported palladium catalysts. A static-circulation reactor study. Catal Lett. 2001;77:135–40.
Takht Ravanchi M, Fadaeerayeni S, Rahimi Fard M. The effect of calcination temperature on physicochemical properties of alumina as a support for acetylene selective hydrogenation catalyst. Res Chem Intermed. 2016;42:4797–811.
Voskanyan PS. Effect of the nature of a support on the catalytic activity of a palladium catalyst in the synthesis of vinyl acetate by gas-phase ethylene acetoxylation. Catal Ind. 2013;5:90–7.
Heck RM, Farauto RJ. Catalytic air pollution control: commercial technology. 2nd ed. New York: Wiley; 2002.
Zheng Q, Farrauto R, Deeba M. Part II: oxidative thermal aging of Pd/Al2O3 and Pd/CexOy-ZrO2 in automotive three way catalysts: the effects of fuel shutoff and attempted fuel rich regeneration. Catalysts. 2015;5:1797–814.
Li M, Weng D, Wu X, Wan J, Wang B. Importance of re-oxidation of palladium by interaction with lanthana for propane combustion over Pd/Al2O3 catalyst. Catal Today. 2013;201:19–24.
Rashidzadeh M, Peyrovi MH, Mondegarian R. Alumina-based supports for automotive palladium catalysts. React Kinet Catal Lett. 2000;69:115–22.
Osaki T, Yamada K, Watari K, Tajiri K, Shima S, Miki T, Tai Y. Palladium–alumina cryogel with high thermal stability and CO oxidation activity. Catal Lett. 2012;142:95–9.
Meusel I, Hoffmann J, Hartmann J, Heemeier M, Bäumer M, Libuda J, Freund H-J. The interaction of oxygen with alumina-supported palladium particles. Catal Lett. 2001;71:5–13.
Chen L, Feng T, Wang P, Xiang Y, Ou B. Catalytic properties of Pd supported on hexaaluminate coated alumina in low temperature combustion of coal mine ventilation air methane. Kinet Catal. 2013;54:767–72.
Demoulin O, Navez M, Ruiz P. The activation of a Pd/γ-alumina catalyst during methane combustion: investigation of the phenomenon and of potential causes. Catal Lett. 2005;103:149–53.
Haack LP, Otto K. X-ray photoelectron spectroscopy of Pd/γ-alumina and Pd foil after catalytic methane oxidation. Catal Lett. 1995;34:31–40.
Weng X, Yuan X, Li H, Li X, Chen M, Wan H. The study of the active surface for CO oxidation over supported Pd catalysts. Sci China Chem. 2015;58:174–9.
Munakata N, Reinhard M. Palladium catalysis for the treatment of contaminated waters: a review. In: Smith JA, Burns SE, editors. Physicochemical groundwater remediation. New York: Kluwer Academic Publishers; 2002. p. 45–71.
Perdigón-Melón JA, Auroux A, Bonnetot B. Calorimetric study of methane interaction with supported Pd catalysts. J Therm Anal Calorim. 2003;72:443–51.
Twigg MV. Catalytic control of emissions from cars. Catal Today. 2011;163:33–41.
Li H, Zhu Q, Li Y, Gong M, Chen Y, Wang J, Chen Y. Effects of ceria/zirconia ratio on properties of mixed CeO2–ZrO2–Al2O3 compound. J Rare Earth. 2010;28:79–83.
Zheng T, He J, Zhao Y, Xia W, He J. Precious metal-support interaction in automotive exhaust catalysts. J Rare Earth. 2014;32:97–107.
Satsuma A, Osaki K, Yanagihara M, Ohyama J, Shimizu K. Activity controlling factors for low-temperature oxidation of CO over supported Pd catalysts. Appl Catal B-Environ. 2013;132–133:511–8.
Li K, Wang X, Zhou Z, Wu X, Weng D. Oxygen storage capacity of Pt-, Pd-, Rh/CeO2-based oxide catalyst. J Rare Earth. 2007;25:6–10.
Alikin EA, Vedyagin AA. High temperature interaction of rhodium with oxygen storage component in three-way catalysts. Top Catal. 2016;59:1033–8.
Widmann D, Behm RJ. Active oxygen on a Au/TiO2 catalyst: formation, stability, and CO oxidation activity. Angew Chem Int Ed. 2011;50:10241–5.
Fessi S, Ghorbel A. Preparation of alumina supported palladium catalysts by sol-gel method. J Sol-Gel Sci Technol. 2000;19:417–20.
Zheng X, Chen X, Chen J, Zheng Y, Jiang L. Synthesis and application of highly dispersed ordered mesoporous silicon-doped Pd-alumina catalyst with high thermal stability. Chem Eng J. 2016;297:148–57.
Wang Q, Li G, Zhao B, Zhou R. The effect of rare earth modification on ceria–zirconia solid solution and its application in Pd-only three-way catalyst. J Mol Catal A-Chem. 2011;339:52–60.
Haneda M, Kintaichi Y, Nakamura I, Fujitani T, Hamada H. Effect of surface structure of supported palladium catalysts on the activity for direct decomposition of nitrogen monoxide. J Catal. 2003;218:405–10.
Soni KC, Krishna R, Chandra Shekar S, Singh B. Catalytic oxidation of carbon monoxide over supported palladium nanoparticles. Appl Nanosci. 2016;6:7–17.
Légaré P, Finck F, Roche R, Maire G. Palladium particles growth on various aluminas. In: Chapon C, Gillet MF, Henry CR, editors. Small particles and inorganic clusters. Berlin: Springer; 1989. p. 19–22.
Shubin VE, Shvetz VA, Savel’eva GA, Popova NM. EPR study of Pd+ ions in palladium–alumina catalysts and their interaction with carbon monoxide and oxygen. Kinet Catal. 1982;23:1153–60 (in Russian).
Sass AS, Shvetz VA, Savel’eva GA, Popova NM, Kazanskii VB. EPR study of palladium and platinum ions in Pd/MgO and Pt/MgO catalysts. Kinet Catal. 1983;24:1167–72 (in Russian).
Sass AS, Shvetz VA, Savel’eva GA, Popova NM, Kazanskii VB. Reactivity of O2 − anion-radicals and mechanism of low-temperature oxidation of carbon monoxide on Ce/Al2O3 and Ce-Pd/Al2O3. Kinet Catal. 1985;26:924–31 (in Russian).
Chen X, Schwank JW, Fisher GB, Cheng Y, Jagner M, McCabe RW, Katz MB, Graham GW, Pan X. Nature of the two-step temperature-programmed decomposition of PdO supported on alumina. Appl Catal A-Gen. 2014;475:420–6.
Cao Y, Ran R, Wu X, Zhao B, Wan J, Weng D. Comparative study of ageing condition effects on Pd/Ce0.5Zr0.5O2 and Pd/Al2O3 catalysts: catalytic activity, palladium nanoparticle structure and Pd-support interaction. Appl Catal A-Gen. 2013;457:52–61.
Matam SK, Otal EH, Aguirre MH, Winkler A, Ulrich A, Rentsch D, Weidenkaff A, Ferri D. Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal Today. 2012;184:237–44.
Misono M. Recent progress in the practical applications of heteropolyacid and perovskite catalysts: catalytic technology for the sustainable society. Catal Today. 2009;144:285–91.
Vedyagin AA, Volodin AM, Stoyanovskii VO, Mishakov IV, Medvedev DA, Noskov AS. Characterization of active sites of Pd/Al2O3 model catalysts with low Pd content by luminescence, EPR and ethane hydrogenolysis. Appl Catal B-Environ. 2011;103:397–403.
Zhou Y, Wang Z, Liu C. Perspective on CO oxidation over Pd-based catalysts. Catal Sci Technol. 2015;5:69–81.
Vedyagin AA, Gavrilov MS, Volodin AM, Stoyanovskii VO, Slavinskaya EM, Mishakov IV, Shubin YV. Catalytic purification of exhaust gases over Pd–Rh alloy catalysts. Top Catal. 2013;56:1008–14.
Vedyagin AA, Volodin AM, Stoyanovskii VO, Kenzhin RM, Slavinskaya EM, Mishakov IV, Plyusnin PE, Shubin YV. Stabilization of active sites in alloyed Pd–Rh catalysts on γ-Al2O3 support. Catal Today. 2014;238:80–6.
Boehm HP, Knözinger H. In: Anderson JR, Boudart M, editors. Catalysis-science and technology, vol. IV. Berlin: Springer; 1983. p. 39–209.
Powder Diffraction File. PDF-2/Release 2009: International Centre for Diffraction Data. USA.
Vedyagin AA, Volodin AM, Kenzhin RM, Chesnokov VV, Mishakov IV. CO Oxidation over Pd/ZrO2 catalysts: role of support′s donor sites. Molecules. 2016;21:1289.
Lieske H, Volter J. Palladium redispersion by spreading of palladium(II) oxide in oxygen treated palladium/alumina. J Phys Chem. 1985;89:1841–2.
Lieske H, Lietz G, Hanke W, Völter J. Oberflächenchemie, Sintern und Redispergieren von Pd/Al2O3-Katalysatoren. Z Anorg Allg Chem. 1985;527:135–49.
Juszczyk W, Karpinski Z, Ratajczykowa I, Stanasiuk Z, Zielinski J, Sheu LL, Sachtler WMH. Characterization of supported palladium catalysts: III. PdAl2O3. J Catal. 1989;120:68–77.
Pawlonka J, Gac W, Greluk M, Słowik G. Application of microemulsion method for development of methanol steam reforming Pd/ZnO catalysts. J Therm Anal Calorim. 2016;125:1265–72.
Sicolo S, Pacchioni G. Charging and stabilization of Pd atoms and clusters on an electron-rich MgO surface. Surf Sci. 2008;602:2801–7.
Gaspar AB, Dieguez LC. Dispersion stability and methylcyclopentane hydrogenolysis in Pd/Al2O3 catalysts. Appl Catal A-Gen. 2000;201:241–51.
Tessier D, Rakai A, Bozon-Verduraz F. Spectroscopic study of the interaction of carbon monoxide with cationic and metallic palladium in palladium–alumina catalysts. J Chem Soc Faraday Trans. 1992;88:741–9.
Ciuparu D, Bensalem A, Pfefferle L. Pd–Ce interactions and adsorption properties of palladium: CO and NO TPD studies over Pd–Ce/Al2O3 catalysts. Appl Catal B-Environ. 2000;26:241–55.
Nilsson PO. Optical properties of PdO in the range of 0.5–5.4 eV. J Phys C Solid State Phys. 1979;12:1423–7.
Acknowledgements
This work was supported by Russian Academy of Sciences and Federal Agency of Scientific Organizations (state-guaranteed order for BIC, Project Number 0303-2016-0014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vedyagin, A.A., Volodin, A.M., Kenzhin, R.M. et al. Characterization and study on the thermal aging behavior of palladium–alumina catalysts. J Therm Anal Calorim 130, 1865–1874 (2017). https://doi.org/10.1007/s10973-017-6530-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6530-y