Abstract
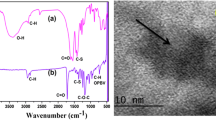
The poly(ε-caprolactone) (PCL) was synthesized by ring-opening polymerization at 160 °C under nitrogen atmosphere for 2 h by bulk polymerization method in the presence of barium thioglycolate (Ba-TG) as an initiator and stannous octoate as a catalyst. The monomer-to-initiator ratio was maintained at 400. The Ba-TG end-capped PCL was characterized by various analytical tools like FTIR spectroscopy, NMR spectroscopy, AFM, DSC, TGA, POM and HRTEM. The non-isothermal crystallization kinetic study was executed with the help of DSC in order to determine the rate at which nucleation formation and spherulitic growth take place. The thermal degradation kinetic studies were performed with the help of TGA to know the degradation rate as well as energy of activation (Ea) using different non-isothermal kinetic models. The main aim of the present investigation is to determine the role of chain end-capping agent (Ba-TG) on the crystallization and degradation process of PCL. It was found that the Ba-TG induced the 3D spherulitic crystal growth of PCL.










Similar content being viewed by others
References
Salgado CL, Sanchez EMS, Zavaglia CAC, Granja PL. Bio-compatibility and bio-degradability of poly(caprolactone)-sebacic acid blended gels. J Biomed Mat Res Part A. 2012;100A:243–51.
Zairs S, Brown TD, Reichert JC, Berner A. Poly(caprolactone) scaffolds fabricated by melt electrospinning for bone tissue engineering. Materials. 2016;9:232–46.
Jiang L, Lou L, Sun W, Xu L, Shen Z. Ring opening polymerization of caprolactone with a divalent samarium bis(phosphido) complex. J Appl Polym Sci. 2005;98:1558–64.
Dobizyski P, Li S, Kaspersczyk J, Bero M, Gasc F, Vert M. Structure-property relationship of copolymer obtained by ring opening polymerization of glycolide and caprolactone, part-1. Synthesis and characterization. Biomacromolecules. 2005;6:483–8.
Contreras JM, Medina D, Carrasquero FL, Contreras RB. Ring opening polymerization of caprolactone initiated by samarium acetae. J Polym Res. 2013;20:244–50.
Monelave M, Contreras JM, Laredo E, Carrasquero FL. Ring opening polymerization of (R, S)-β-butyrolactone and caprolactone using sodiumhydride as initiator. Express Polym Lett. 2010;7:431–41.
Sivabalan A, Meenarathi B, Palanikumar S, Anbarasan R. Synthesis and characterization of poly(caprolactone): a comparative study. Int J Sci Res Eng Technol. 2014;1:9–14.
Cayuela J, Legare VB, Cassagnou P, Michel A. Ring opening polymerization of caproalctone initiated with titanium n-propoxide or titanium phenoxide. Macromolecules. 2006;39:1338–46.
Kannammal L, Palanikumar S, Meenarathi B, Anbarasan R. Synthesis, characterization and band gap study of calcium mercaptosuccinate. J Thermoplast Compos Mater. 2017;30:1056–68.
Meenarathi B, Siva P, Palanikumar S, Kannammal L, Anbarasan R. Synthesis, characterization and drug release activity of poly(caprolactone)/Fe3O4 nanocomposites. Nanocomposites. 2016;2:98–107.
Rajkumar B, Dhanalakshmi T, Meenarathi B, Anbarasan R. Synthesis and characterization of novel fluorescent amphiphilic diblock copolymers. Polym Bull. 2016;73:2147–63.
Jeyapriya M, Meenarathi B, Anbarasan R. Synthesis, characterization, catalytic activity and splinting activity of nano Ag end capped l-glutathione bridged amphiphilic diblock copolymer. J Appl Polym Sci. 2016;133:1–11.
Murugesan A, Meenarathi B, Kannammal L, Anbarasan R. Synthesis, characterization and application of poly(sulfanilicacid) based triblock copolymer. Adv Polym Technol. 2016;26:1–9.
Kailash S, Meenarathi B, Palanikumar S, Anbarasan R. Synthesis, characterization, drug delivery and splinting activity of folic acid bridged poly(caprolactone-co-tetrahydrofuran). Int J Polym Mater Polym Biomater. 2015;64:620–7.
Sowkath A, Ahmad M, Anbarasan R. Ring opening polymerization ε-caprolactone by Schiff base metal complexes. Int J Chem Biol Sci. 2014;1:1–12.
Chrissafis K, Antoniadis G, Paraskevopoulos KM, Vassiliou A, Bikiaris DN. Comparative study of the effect of different nanoparticles on the mechanical properties and thermal degradation mechanism of in situ prepared poly(e-caprolactone) nanocomposites. Compos Sci Technol. 2007;67:2165–74.
Nanaki SG, Papageorgiou GZ, Bikiaris DN. Crystallization of novel poly(ε-caprolactone)-block-poly(propyleneadipate) copolymers. J Therm Anal Calorim. 2012;108:633–45.
Luduen L, Vazquez A, Alvarez V. Viscoelastic behavior of poly(caprolactone)/clay nanocomposites. J Compos Mater. 2012;46:677–89.
Gopinathan J, Pillai M, Elakkiy V, Selvakumar R, Bhattacharyya A. Carbon nanofillers incorporated electrically conducting poly(caprolactone) nanocomposite films and their biocompatibility studies using MG-63 cell line. Polym Bull. 2016;73:1037–53.
Wng XL, Huang FY, Zhou Y, Wang YZ. Non-isothermal crystallization kinetics of poly(ε-caprolactone)/montmorillonite nanocomposites. J Macromol Sci Part B Phys. 2009;48:710–22.
Saeed K, Park SY. Preparation and properties of poly(caprolactone)/poly(butylenes terephthalate) blend, Iran. J Chem Eng. 2010;29:77–81.
Kawazu K, Nakagawa S, Ishizone T, Nojima S, Arai D, Yamaguchi K, Nakahama S. Effects of bulky end groups on the crystallization kinetics of poly(caprolactone) homopolymers confined in a cylindrical nanodomain. Macromolecules. 2017;50:7202–10.
Huo H, Yang Y, Zhao X. Effects of lithium perchlorate on the nucleation and crystallization of PEO and PCL in the PEO–PCL–lithium perchlorate ternary blend. CrystEngComm. 2014;16:1351–8.
Jenkins MJ, Harrison KL. The effect of molecular weight on the crystallization kinetics of poly(caprolactone). Polym Adv Technol. 2006;17:474–8.
Marquez Y, Franco L, Puiggali J. Thermal degradation studies of poly(trimetylene carbonate) blends with either polylactide or poly(caprolactone). Thermochim Acta. 2012;550:65–75.
Peng H, Han Y, Liu T, He C. Morphology and thermal degradation behaviour of highly exfoliated Co–Al layered double hydroxide/poly(caprolactone) nanocomposites prepared by simple solution intercalation. Thermochim Acta. 2010;502:1–7.
Nanaki SG, Chuissafis K, Bikiaris DN. Effect of molar ratio on the mass loss kinetics of poly(caprolactone-co-propyleneadipate) copolymers. Thermochim Acta. 2011;517:45–52.
Carmoona VB, Compos AD, Marcocini JM, Mattoso C. Kinetics of thermal degradation applied to biocomposites with TPS, PCL and sisal fibers by non-isothermal procedures. J Thermal Anal Calorim. 2014;115:153–60.
Joshi P, Madras G. Degradation of poly(caprolactone) in supercritical fluids. Polym Degrad Stab. 2008;93:1901–8.
Maiti ASN, Jacob J. Non-isothermal crystallization and micro-structural behaviour of poly(caprolactone) and tapioca starch based bio-composites. Int J Polym Anal Charact. 2017;22:222–36.
Jancirani A, Kohila V, Meenarathi B, Yellilarasi A, Anbarasan R. Synthesis, characterization and non-isothermal degradation kinetics of poly(monoethyleneglycol dimethacrylate-co-4-aminobenzoate). Bull Mater Sci. 2016;39:1725–33.
Terzopoulou Z, Baciu D, Gounari E, Steriotis T, Bikiaris D. Biocompatible nanobiogels reinforced poly(caprolactone) composites synthesized via insitu ring opening polymerization. Polymers. 2018;10:381–407.
Roumeli E, Papageorgiou DG, Tsanaktsis V, Terzopoulou Z, Chrissafis K, Bikiaris DN. Amino functionalized multiwalled carbon nanotube lead to successful ring opening polymerization of poly(caprolactone): enhanced interfacial bonding and optimized mechanical properties. Appl Mater Interfaces. 2015;7:11683–94.
Terzopoulou Z, Papageorgiou DG, Papageorgiou GZ, Bikiaris DN. Effect of surface functionalization of halloysite nanotubes on synthesis and thermal properties of ply(caproalctone). J Mater Sci. 2018;53:6519–41.
Vassiliou AA, Papageorgiou GZ, Achilias DS, Bikiaris DN. Non-isothermal crystallization kinetics of insitu prepared poly(caprolactone)/surface treated SiO2 nanocomposites. Macromol Chem Phys. 2007;208:364–76.
Lin L, Xu Y, Qin J, Wang S, Xiao M, Meng Y. Correlation between crystallization behavior and mechanical properties of biodegradable poly(caprolactone-co-cyclohexene carbonate). Polym Plast Technol Eng. 2017;42:1–12.
Nerantzaki M, Papageorgiou GZ, Bikiaris DN. Effect of nanofillers type on the thermal properties and enzymatic degradation of poly(caprolactone). Polym Degrad Stab. 2014;108:257–68.
Papageorgiou DG, Roumeli E, Terzopoulou Z, Tsanaktsis V, Chrissafis K, Bikiaris DN. Poly(caproalctone)/MWCNT nanocomposites prepared by insitu ring opening polymerization: decomposition property using thermogravimetric analysis and analytical pyrolysis gas chromatography/mass spectroscopy. J Anal Appl Pyrolysis. 2015;115:125–31.
Terzopoulou Z, Bikiaris DN, Potsi G, Gournis D, Papageorgiou GZ, Rudolf P. Mechanical, thermal and decomposition behaviour of poly(caprolactone) nanocomposites with clay supported carbon nanotube hybrids. Thermochim Acta. 2016;642:67–80.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahalakshmi, S., Alagesan, T., Parthasarathy, V. et al. Non-isothermal crystallization kinetics and degradation kinetics studies on barium thioglycolate end-capped poly(ε-caprolactone). J Therm Anal Calorim 135, 3129–3140 (2019). https://doi.org/10.1007/s10973-018-7514-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7514-2