Abstract
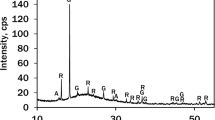
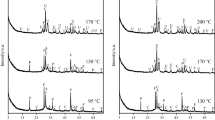
In this work, the possibility of applying silica gel waste for the production of wollastonite via both single- and two-step methods was investigated. For the synthesis of wollastonite, two initial mixtures with CaO/SiO2 molar ratio of 0.75 and 1.0 were prepared. The synthesis was done by single-step and two-step methods: one-step synthesis was carried out in 800–950 °C temperature range in high-temperature furnace, while two-step method was based on hydrothermal treatment in autoclave (w/s-10, 200 °C, 4–72 h) and calcination of obtained products (800–950 °C, 1 h). It was determined that by using single-step synthesis, only the negligible amount of wollastonite was formed. The different results were obtained after calcination of hydrothermal synthesis products at 850 °C temperature, because wollastonite was the main compound. The obtained results were confirmed by data obtained from the XRD and STA analyses and thermodynamic calculations.









Similar content being viewed by others
References
Malaiškienė J, Vaičienė M, Žurauskienė R. Effectiveness of technogenic waste usage in products of building ceramics and expanded clay concrete. Constr Build Mater. 2011;25:3869–77.
Nour WMN, Mostafa AA, Ibrahim DM. Recycled wastes as precursor for synthesizing wollastonite. Ceram Int. 2008;34:101–5.
Yao X, Wang W, Liu M, Yao Y, Wu S. Synergistic use of industrial solid waste mixtures to prepare ready-to-use lightweight porous concrete. J Clean Prod. 2019;211:1034–43.
El-Didamony H, Amer AA, Mohammed MS, El-Hakim MA. Fabrication and properties of autoclaved aerated concrete containing agriculture and industrial solid wastes. J Build Eng. 2019;22:528–38.
Başaran C, Canikoğlu N, Toplan HÖ, Toplan N. The crystallization kinetics of the MgO–Al2O3–SiO2–TiO2 glass ceramics system produced from industrial waste. J Therm Anal Calorim. 2016;125(2):695–701.
Başaran C, Toplan HÖ, Toplan N. The crystallization kinetics of the Bi2O3-added MgO–Al2O3–SiO2–TiO2 glass ceramics system produced from industrial waste. J Therm Anal Calorim. 2018;134(1):313–21.
Iljina A, Baltakys K, Bankauskaite A, Eisinas A, Kitrys S. The stability of formed CaF2 and its influence on the thermal behavior of C–S–H in CaO–silica gel waste-H2O system. J Therm Anal Calorim. 2017;127(1):221–8.
Mermer NK, Yilmaz MS, Odzemir OD, Piskin MB. The synthesis of silica-based aerogel from gold mine waste for thermal insulation. J Therm Anal Calorim. 2017;129(3):1807–12.
World Bank official website. https://data.worldbank.org/indicator/EN.ATM.CO2E.KT. Accessed 21 Feb 2019.
Ashraf W, Olek J, Tian N. Multiscale characterization of carbonated wollastonite paste and application of homogenization schemes to predict its effective elastic modulus. Cem Concr Compd. 2016;72:284–98.
Scalenghe R, Malucelli F, Ungaro F, Perazzone L, Filippi N, Edwards AC. Influence of 150 years of land use on anthropogenic and natural carbon stocks in Emilia-Romagna region (Italy). Environ Sci Technol. 2011;14:5112–7.
Andrew RM. Global CO2 emissions from cement production. Earth Syst Sci Data. 2018;10:195–217.
Nguyen L, Moseson AJ, Farnam Y, Spatari S. Effects of composition and transportation logistics on environmental, energy and cost metrics for the production of alternative cementitious binders. J Clean Prod. 2018;185:628–45.
Juenger MCG, Winnefeld F, Provis JL, Ideker JH. Advances in alternative cementitious binders. Cem Concr Res. 2011;41(12):1232–43.
Jo B, Chakraborty S, Jo JH. Effectiveness of a hydrothermally produced alternative cementitious material on the physical and mechanical performance of concrete. J Clean Prod. 2017;142(4):3269–80.
Naraharisetti PK, Yeo TY, Bu J. New classification of CO2 mineralization processes and economic evaluation. Renew Sust Energ Rev. 2019;99:220–33.
Ashraf W. Carbonation of cement-based materials: challenges and opportunities. Constr Build Mater. 2016;120:558–70.
Ashraf W, Olek J. Elucidating the accelerated carbonation products of calcium silicates using multi-technique approach. J CO2 Util. 2018;12:61–74.
Gartner E, Sui T. Alternative cement clinkers. Cem Concr Res. 2018;144:27–39.
UN Environment, Scrivener KL, John VM, Gartner E. Eco-efficient cements: potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res. 2018;114:2–26.
Ashraf W, Olek J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: potential of utilizing low-lime calcium silicates in cement-based materials. J Mater Sci. 2016;51:6173–91.
Thompson SP, Day SJ, Parker JE, Evans A, Tang CC. Fine-grained amorphous calcium silicate CaSiO3 from vacuum dried sol–gel—production, characterisation and thermal behavior. J Non Cryst Solids. 2012;358(5):885–92.
Voicu G, Ene VL, Sava DF, Surdu VA, Busuioc C. Sol–gel derived vitroceramic materials for biomedical applications. J Non Cryst Solids. 2016;449:75–82.
Li HC, Wang DG, Chen CZ. Effect of zinc oxide and zirconia on structure, degradability and in vitro bioactivity of wollastonite. Ceram Int. 2015;41(8):10160–9.
Solonenko AP, Blesman AI, Polonyakin DA. Preparation and in vitro apatite-forming ability of hydroxyapatite and β-wollastonite composite materials. Ceram Int. 2018;44(15):17824–34.
Hu Y, Xiao Z, Wang H, Ye C, Wu Y, Xu S. Fabrication and characterization of porous CaSiO3 ceramics. Ceram Int. 2019;45(3):3710–4.
Kurczyk HG. Synthetic diopside and wollastonite—new raw materials for ceramic. Ceramurg Int. 1978;8:128–40.
Kotsis I, Balogh A. Synthesis of wollastonite. Ceram Int. 1989;15:79–85.
Ismail H, Shamsudin R, Hamid MAA. Effect of autoclaving and sintering on the formation of β-wollastonite. Mater Sci Eng C. 2016;58:1077–81.
Pei LZ, Yang LJ, Yang Y, Fan CG, Yin WI, Chen J, Zhang QF. A green and facile route to synthesize calcium silicate nanowires. Mater Charact. 2010;61:1281–5.
Yazdani A, Rezaie HR, Ghassai H. Investigation of hydrothermal synthesis of wollastonite using silica and nano silica at different pressures. J Ceram Process Res. 2010;11(3):348–53.
Vichapund S, Kitiwan M, Atong D, Thavorniti P. Microwave synthesis of wollastonite powder from eggshells. J Eur Ceram Soc. 2011;31:2435–40.
Leite FHG, Almeida TF, Faria RT, Holanda JNF. Synthesis and characterization of calcium silicate insulating material using avian eggshell waste. Ceram Int. 2017;43:4674–9.
Wang Y, Song J, Guo Q, Xi X, Hou G, Wei G, Qu J. The environmental sustainability of synthetic wollastonite using waste from zirconium oxychloride production. J Clean Prod. 2018;172:2576–84.
Palakurthy S, Reddy VG, Samudrala RK, Azeem A. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater Sci Eng C. 2019;98:109–17.
Heriyanto, Pahlevani F, Sahajwalla V. Synthesis of calcium silicate from selective thermal transformation of waste glass and waste shell. J Clean Prod. 2018;172:3019–27.
Almasri KA, Sidek HAA, Matori KA, Zaid MHM. Effect of sintering temperature on physical, structural and optical properties of wollastonite based glass–ceramic derived from waste soda lime silica glasses. Results Phys. 2017;7:2242–7.
Almeida TF, Leite FHG, Faria RT, Holanda JNF. Thermal study of calcium silicate material synthesized with solid wastes. J Therm Anal Calorim. 2017;128(3):1265–72.
Baltakys K, Iljina A, Bakkauskaite A. Thermal properties and application of silica gel waste contaminated with F– ions for C–S–H synthesis. J Therm Anal Calorim. 2015;121(1):145–54.
LTD “Alufluor AB” Official Website. http://www.lifosa.com/en/products-and-services. Accessed 21 Feb 2019.
Rudelis V, Dambrauskas T, Grineviciene A, Baltakys K. The prospective approach for the reduction of fluoride ions mobility in industrial waste by creating products of commercial value. Sustainability. 2019;11:634–52.
Dambrauskas T, Baltakys K, Eisinas A. Formation and thermal stability of calcium silicate hydrate substituted with Al3+ ions in the mixtures with CaO/SiO2 = 1.5. J Therm Anal Calorim. 2018;131:501–12.
Standard thermodynamic properties of chemical substances. CRC Press LLC; 2000.
Baltakys K, Siauciunas R. Gyrolite formation in CaO–SiO2 center dot nH(2)O-gamma-Al2O3–Na2O–H2O system under hydrothermal conditions. Pol J Chem. 2007;81(1):103–14.
Dousti A, Beaudoin JJ, Shekarchi M. Chloride binding in hydrated MK, SF and natural zeolite–lime mixtures. Constr Build Mater. 2017;154:1035–47.
Acknowledgements
This research was funded by a Grant (No. MIP – 17-92) from the Research Council of Lithuania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gineika, A., Baltakys, K. & Dambrauskas, T. The application of silica gel waste for the two-step synthesis of wollastonite in temperature range of 200–950 °C. J Therm Anal Calorim 138, 2263–2273 (2019). https://doi.org/10.1007/s10973-019-08481-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08481-5