Abstract
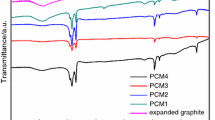
In this paper, sodium sulfate decahydrate (SSD) with a phase transition temperature of 32 °C was selected as the phase change energy storage material. However, SSD has the problems of large degree of supercooling, obvious phase stratification, and low thermal conductivity. To address these issues, a new SSD composite phase change energy storage material was synthesized by adding certain amounts of nucleating agents, thickeners, and high thermal conductivity agent to SSD. The results showed that when the addition of nucleating agent Na2B4O7·10H2O and thickener carboxymethyl cellulose (CMC) reached an optimal formulation ratio of SSD:Na2B4O7·10H2O:CMC = 100:5:3, the supercooling degree of the SSD composite phase change material was only 1.5 °C. Moreover, the heat release time of the phase change process was 25 min, and no phase stratification occurred. When expanded graphite with high thermal conductivity was added with the mass ratio of 9%, the effect on the supercooling degree of the SSD composite energy storage material was small, and the thermal conductivity increased by 3.9 times, reaching 2.13 W m−1 K−1. Differential scanning calorimetry method and step cooling curve method were used to test the decay rate of the thermal properties of the new composite phase change energy storage material, and the results were relatively close.



















Similar content being viewed by others
References
Wu H, Diao W, Ding R, et al. Overview of the development status and application prospects of phase change energy storage materials. Build Dev. 2009;8:92–5.
Telkes M. Composition of matter for the storage of heat. US Patent 2677664; 1951.
Telkes M. Thixotropic mixture and method of making same. US Patent 3986969; 1976.
Telkes M. Solar energy storage. ASHRAE J. 1974;9:38–44.
Kong X. Study on the performance of phase change material combined with building envelop for cold storage. Tianjin: Tianjin University; 2013.
Sandnes B, Rekstad J. Super cooling salt hydrates: stored enthalpy as a function of temperature. Sol Energy. 2006;80:616–25.
Xu J, Ke X. Study of phase change property of sodium acetate trihydrate as energy storage material. Mater Rev. 2007;21(9):319–21.
Chen M. Preparation and properties of inorganic salt/expanded graphite composite phase change thermal storage materials. Guangzhou: South China University of Technology; 2005.
Carlsson B. Phase change behaviour of some latent heat storage media based on calcium chloride hexahydrate. Sol Energy. 2009;83(4):485–500.
Yan Q, He W. Application of phase change energy storage technology. Mater Rev. 2014;28(24):209–12.
Xiaofeng X, Xuelai Z, Sunxi Z, et al. Experimental and application study of Na2SO4·10H2O with additives for cold storage. J Therm Anal Calorim. 2019;136:505–12.
Zhang D, Gu H, Lv G, et al. Experimental study on the undercooling of the sodium acetate solution. Guangxi Phys. 2015;36(2):24–6.
Li F, Wang P, et al. The preparation of phase change material Na2SO4·10H2O with the phase transition temperature at room temperature. Synth Mater Aging Appl. 2015;44(1):39–46.
Lu D, Hu P, Zhao B, et al. Study on the performance of nanoparticles as nucleating agents for sodium acetate trihydrate. J Eng Thermophys. 2012;33(8):1279–82.
Vishnuprasad S, Bindusai M, Lakshmi MN, et al. Experimental investigation on the thermophysical properties of beryllium oxide-based nanofluid and nano-enhanced phase change material. J Therm Anal Calorim. 2019;137:1527–36.
Meshing H, Cabeza LF. Heat and cold storage with PCM. Berlin: Acid-Free Paper; 2008.
Liu L. Study on improving the service life of inorganic phase change energy storage materials. Tianjin: Hebei University of Technology; 2005.
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (No. 51708551) for the support of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, X., Mao, J., Geng, S. et al. Study on performance optimization of sodium sulfate decahydrate phase change energy storage materials. J Therm Anal Calorim 143, 3923–3934 (2021). https://doi.org/10.1007/s10973-020-09306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09306-6