Abstract
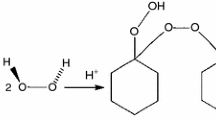
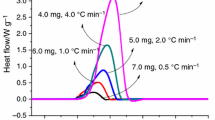
Cosmeceutical products have improved over the years; however, the explosions and fire accidents in cosmeceutical factories worldwide have not ceased. Literature researches on this genre of potential risk have been seldom published. Cosmeceutical benzoyl peroxide (CBPO) is usually used on facial products, especially for acne treatment. The thermal stability of CBPO with additions and calorimetric technology is utilized to obtain the thermal stability and reaction characteristics. Depending on the experimental thermal stability parameters, apparent activation energy was calculated by various integral and differential kinetic models. Uniting the isoconversional kinetic analysis and numerical simulation of the isothermal condition can better realize the decomposition characteristics and potential process risk. Dozens of milligrams of CBPO were dunked with 10,000 ppm of Cu, Fe, and Zn to inspect the exothermal behavior. The findings can be a reference for the essential safety parameters and customer safety design for the database.








Similar content being viewed by others
Abbreviations
- ∆H d :
-
Heat of decomposition (J g‒1)
- A :
-
Frequency factor (1 s‒1)
- C :
-
Constant (dimensionless)
- C s :
-
Constant for Starink method (dimensionless)
- D :
-
Correction coefficient for apparent activation energy (dimensionless)
- E a :
-
Apparent activation energy (kJ mol‒1)
- f(α):
-
Differential form of reaction mechanism function (dimensionless)
- g(α):
-
Integral form of reaction mechanism function (dimensionless)
- i :
-
The power of absolute temperature in Starink (dimensionless)
- p(x):
-
Integral temperature
- R :
-
Universal gas constant [8.314 J (mol K)‒1]
- R 2 :
-
Coefficient of determination (dimensionless)
- SADT :
-
Self-accelerating decomposition temperature (°C)
- t :
-
Time (min)
- T :
-
Absolute temperature (K)
- T 0 :
-
Apparent onset temperature (°C)
- TCL :
-
Time to conversion limit (day)
- TMR iso :
-
Time to the maximum rate at isothermal conditions (h)
- T f :
-
Final reaction temperature (°C)
- T p :
-
Peak temperature (°C)
- α :
-
Extent of conversion (dimensionless)
- β :
-
Heating rate (°C min‒1)
References
Lin T, Zhang M, Xu F, Wang X, Xu Z, Guo L. Colorimetric detection of benzoyl peroxide based on the etching of silver nanoshells of Au@Ag nanorods. Sens Actuators B. 2018;261:379–84.
Lu KT, Chen TC, Hu KH. Investigation of the decomposition reaction and dust explosion characteristics of crystalline benzoyl peroxides. J Hazard Mater. 2009;161:246–56.
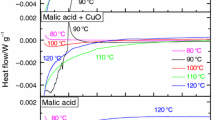
Huang AC, Chuang YK, Huang CF, Shu CM. Thermokinetic analysis of the stability of malic and salicylic acids in cosmeceutical formulations containing metal oxides. J Therm Anal Calorim. 2017;132:165–72.
Zhang Y, Chen R, Zhao M, Luo J, Feng W, Fan W, et al. Hazard evaluation of explosion venting behaviours for premixed hydrogen-air fuels with different bursting pressures. Fuel. 2020;268:117313.
Huang AC, Chen WC, Huang CF, Zhao JY, Deng J, Shu CM. Thermal stability simulations of 1,1-bis(tert-butylperoxy)-3,3,5 trimethylcyclohexane mixed with metal ions. J Therm Anal Calorim. 2017;130:949–57.
Liu SH, Hou HY, Shu CM. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76.
Cao CR, Liu SH, Das M, Shu CM. Evaluation for the thermokinetics of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf Environ Prot. 2018;117:426–38.
Cao CR, Liu SH, Huang AC, Lee MH, Ho SP, Yu WL, et al. Application of thermal ignition theory of di(2,4-dichlorobenzoyl) peroxide by kinetic-based curve fitting. J Therm Anal Calorim. 2018;133:753–61.
Huang AC, Huang CF, Xing ZX, Jiang JC, Shu CM. Thermal hazard assessment of the thermal stability of acne cosmeceutical therapy using advanced calorimetry technology. Process Saf Environ Prot. 2019;131:197–204.
Huang AC, Huang CF, Tang Y, Xing ZX, Jiang JC. Evaluation of multiple reactions in dilute benzoyl peroxide concentrations with additives using calorimetric technology. J Loss Prev Process Ind. 2021;69:104373.
Tan J, Bissonnette R, Gratton D, Kerrouche N, Canosa JM. The safety and efficacy of four different fixed combination regimens of adapalene 0.1%/benzoyl peroxide 2.5% gel for the treatment of acne vulgaris: results from a randomised controlled study. Eur J Dermatol. 2018;28:502–8.
Osman-Ponchet H, Sevin K, Gaborit A, Wagner N, Poncet M. Fixed-combination gels of adapalene and benzoyl peroxide provide optimal percutaneous absorption compared to monad formulations of these compounds: results from two in vitro studies. Dermatol Ther (Heidelb). 2017;7:123–31.
You ML. Thermal hazard evaluation of cumene hydroperoxide-metal ion mixture using DSC, TAM III, and GC/MS. Molecules. 2016;21:562.
Duh YS. Chemical kinetics on thermal decompositions of cumene hydroperoxide in cumene studied by calorimetry: an overview. Thermochim Acta. 2016;637:102–9.
Wang Q, Liu SH, Huang AC, Huang CF, Chuang YK, Shu CM. Effects of mixing malic acid and salicylic acid with metal oxides in medium- to low-temperature isothermal conditions, as determined using the thermal activity monitor IV. J Therm Anal Calorim. 2018;133:779–84.
Wei M, Huang AC, Shu CM, Zhang L. Thermal decomposition and nonisothermal kinetics of monoethanolamine mixed with various metal ions. Sci Rep. 2019;9:1592.
Yang Y, Tsai YT, Zhang YN, Shu CM, Deng J. Inhibition of spontaneous combustion for different metamorphic degrees of coal using Zn/Mg/Al–CO3 layered double hydroxides. Process Saf Environ Prot. 2018;113:401–12.
Tsai YT, Yang Y, Huang HC, Shu CM. Inhibitory effects of three chemical dust suppressants on nitrocellulose dust cloud explosion. AlChE J. 2020;66:e16888.
Tsai YT, Yang Y, Wang CP, Shu CM, Deng J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int J Energy Res. 2018;42:1158–71.
Shiue GY, Huang AC, Chen JR. Thermal decomposition of triacetone triperoxide by differential scanning calorimetry. J Therm Anal Calorim. 2018;133:745–51.
Tsai YT, You ML, Qian XM, Shu CM. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard oftert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind Eng Chem Res. 2013;52:8206–15.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S. Kissinger method in kinetics of materials: Things to beware and be aware of. Molecules. 2020;25:2813.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Deng J, Zhao JY, Huang AC, Zhang YN, Wang CP, Shu CM. Thermal behavior and microcharacterization analysis of second-oxidized coal. J Therm Anal Calorim. 2016;127:439–48.
Vyazovkin S, Sbirrazzuoli N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol Rapid Commun. 2006;27:1515–32.
Laiwang B, Liu SH, Shu CM. Thermal hazards of benzoyl peroxide and its derived process products through theoretical thermodynamics assessment and different calorimetric technologies. J Hazard Mater. 2019;380:120891.
Wang J, Jia H, Tang Y, Xiong X, Ding L. Thermal stability and non-isothermal crystallization kinetics of metallocene poly(ethylene–butene–hexene)/high fluid polypropylene copolymer blends. Thermochim Acta. 2017;647:55–61.
Deng J, Zhao JY, Xiao Y, Zhang YN, Huang AC, Shu CM. Thermal analysis of the pyrolysis and oxidation behaviour of 1/3 coking coal. J Therm Anal Calorim. 2017;129:1779–86.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol (Sci Technol). 1971;16:22–31.
Xiao Y, Lü HF, Huang AC, Deng J, Shu CM. A new numerical method to predict the growth temperature of spontaneous combustion of 1/3 coking coal. Appl Therm Eng. 2018;131:221–9.
Laiwang B, Tsai YT, Liu SH, Deng J, Xiao Y, Wang QH, et al. Effects of 1-butyl-3-methylimidazolium nitrate on the thermal hazardous properties of lignitous and long flame coal through a green approach and thermokinetic models. Process Saf Environ Prot. 2019;131:127–34.
Zhang Y, Chung YH, Liu SH, Shu CM, Jiang JC. Analysis of thermal hazards of O, O-dimethylphosphoramidothioate by DSC, TG, VSP2, and GC/MS. Thermochim Acta. 2017;652:69–76.
Malow M, Michael-Schulz H, Wehrstedt KD. Evaluative comparison of two methods for SADT determination (UN H.1 and H.4). J Loss Prev Process Ind. 2010;23:740–4.
Acknowledgements
National Nature Science Foundation of China financially (Nos. 21927815 and 51574046) and National Key Research Development Program of China (No. 2019YFC0810701) supported this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, AC., Liao, FC., Huang, CF. et al. Calorimetric approach to establishing thermokinetics for cosmeceutical benzoyl peroxides containing metal ions. J Therm Anal Calorim 144, 373–382 (2021). https://doi.org/10.1007/s10973-021-10703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10703-8