Abstract
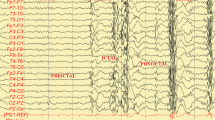
Epileptic encephalopathies (EE) is a term coined by the International League Against Epilepsy (ILAE) to refer to a group of epilepsies in which the ictal and interictal abnormalities may contribute to progressive cerebral dysfunction. Among them, two affect mainly children and are very difficult to deal with, Doose and Lennox-Gastaut syndromes, (DS and LGS, respectively). So far (Zavala-Yoe et al., J Integr Neurosci 15(2):205–223, 2015a and works of ours there), quantitative analysis of single case studies of EE have been performed. All of them are manifestations of drug resistant epileptic encephalopathies (DREES) and as known, such disorders require a lot of EEG studies through all patient’s life. As a consequence, dozens of EEG records are stored by parents and neurologists as time goes by. However, taking into account all this massive information, our research questions (keeping colloquial wording by parents) arise: a) Which zone of the brain has been the most affected so far? b) On which year was the child better? c) How bad is our child with respect to others? We must reflect that despite clinical assessment of the EEG has undergone standardization by establishment of guidelines such as the recently published guidelines of the American Clinical Neurophysiology Society (Tsuchida et al., J Clin Neurophysiol 4(33):301–302, 2016), qualitative EEG will never be as objective as quantitative EEG, since it depends largely on the education and experience of the conducting neurophysiologist (Grant et al., Epilepsy Behav 2014(32):102–107, 2014, Rating, Z Epileptologie, Springer Med 27(2):139–142, 2014). We already answered quantitatively the above mentioned questions in the references of ours given above where we provided entropy curves and an entropy index which encompasses the complexity of bunches of EEG making possible to deal with massive data and to make objective comparisons among some patients simultaneously. However, we have refined that index here and we also offer another two measures which are spatial and dynamic. Moreover, from those indices we also provide what we call a temporal dynamic complexity path which shows in a standard 10–20 system head diagram the evolution of the lowest complexity per brain zone with respect to the EEG period. These results make it possible to compare quantitatively/graphically the progress of several patients at the same time, answering the questions posed above. The results obtained showed that we can associate low spatio-temporal entropy indices to multiple seizures events in several patients at the same time as well as tracking seizure progress in space and time with our entropy path, coinciding with neurophysiologists observations.











Similar content being viewed by others
References
Ahmed MU, Mandic PM (2011) Multivariate multiscale entropy: a tool for complexity analysis of multichannel data. Phys Rev E 84:061918
Andrzejak R et al (2001) Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys Rev E 64(6):8
Bendat J, Piersol A (1966) Measurment and analysis of random data. Wiley, NY, USA
Berg AT, Berkovic SF, Brodie MJ (2010a) Revised terminology and concepts for organization of seizures and epilespies: report of the ILAE Comission on Classification and Terminology, 2005–2009. Epilepsia 51(4):676–685
Berg AT, Brodie MI et al (2010b) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology. Epilepsia 51(4):676–685
Blinowska KJ, Zygierewicz J (2012) Practical biomedical signal analysis using MATLAB. CRC Press, FL, USA
Bruzzo AA, Gesierich B, Santi M, Tassinari CA, Birbaumer N, Rubboli G (2008) Permutation entropy to detect vigilance changes and preictal states from scalp EEG in epileptic patients. A preliminary study. Neurol Sci 29(1):3–9
Chon K, Scully C, Lu S (2009) Approximate entropy for all signals. IEEE Eng Med Biol Mag 0739–5175:18–23
Costa M, Goldberger A, Peng C (2002) Multiscale entropy analysis of complex physiologic time series. Phys Rev 89(6):068,102-1–068,102-4
Devinsky O et al (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 15(3):270–278
Doege C, May T, Michael S, von Spiczak S, Stephani U, Boor R (2013) Myoclonic astatic epilepsy (Doose syndrome)- a lamotrigine responsive epilepsy? Eur J Paediatr Neurol 2013(17): 29–35
Doege C, Kleiss R, Stephani U, von Spiczak S (2014) Myoklonisch-astatische epilepsie. Z Epileptologie, Springer Medizin 2(27): 105–111
Filloux FM (2015) Cannabinoids for pediatric epilepsy? Up in smoke or real science? Trans Pediatr 4(4):271–282
García Ben M, Yohai JV (2004) Quantile-quantile plot for deviance residuals in the generalized linear model. J Comput Graph Stat 13(1):36–47
Gil-Nagel A (2001) Manual de electroencefalografia. McGraw-Hill-Interamericana, Mexico City, Mexico
Grant A, Abdel-Baki S, Weedon J et al (2014) EEG interpretation reliability and interpreter confidence: a large single-centre study. Epilepsy Behav 2014(32):102–107
Hussain S et al (2015) Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy Behav 47(2015):138–141
Jing L, Jiaqing Y, Xianzeng L, Gaoxiang O (2014) Using permutation entropy to measure the changes in eeg signals during absence seizures. Entropy 16(2014):3049–3061
Kipinski L, Konig R, Sieluzycki C, Kordecki W (2011) Application of modern tests for stationarity to single trial MEG data. In: Biological cybernetics. Springer, pp 183–195
Klonowski W (2009) Everything you wanted to ask about EEG but were afraid to get the right answer. Nonlinear Biomed Phys 2(3):1–5
Kwan P, Arimazinoglou A, Berg AT et al (2010) Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Comission on Therapeutic Strategies. Epilepsia 51(6):1069–1077
León A, Davis L, Kraemer H (2011) The role and interpretation of pilot studies in clinical research. J Psychiatr Res 45(5):626–629
Lutz M (2016) Non-adhärenz: klinische erfahrung bestätigt. Neurol Psychiater, Springer 17(10):15–16
Manis G, Nikolopoulos S (2007) Speeding up the computation of approximate entropy. In: 11th Mediterranean conference on medical and biomedical engineering and computing, vol 16
Manuca R et al (1998) Nonstationarity in epileptic EEG and implications for neural dynamics. Math Biosci 1998(147):1–22
Mayer T (2016) Cannabis bei Epilepsie? Hier besteht noch Forschungsbedarf. Neurol Psychiater 17(10):17
Mbuba CK, Ngugi AK, Newton CR, Carter JA (2008) The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia 49:1491–1503
McTague A, Cross JH (2013) Treatment of epileptic encephalopaties. CNS Drugs Springer Int Publ Switzerland 2013(27):174–184
Morinsky D, Green L, Levine D (1986) Concurrent and predictive validity of a self reported measure of medication adherence. Med Care 24:67–74
Nachane DM (2009) Selected problems in the analysis of non-stationary and nonlinear time series. In: IGIDR proceedings/project report series, pp 1–17
Nakamura T, Small M, Hirata Y (2006) Testing for nonlinearity in irregular fluctuations with long-term trends. Phys Rev E 74(026205):026,205-1–026,205-8
Natus Technology (2015) Grass comet plus EEG: lab-based portable EEG systems. Natus Technology, Natus Neurology Incorporated, 1850 Deming Way, Middleton, WI 53562, USA
Neubauer BA, Hahn A (2014) Dooses epilepsien im kindes- und jugendalter. Springer Medizin. Springer, Berlin
Nogués-Solán X, Sorli-Redó M, Villar-García J (2007) Instrumentos de medida de adherencia al tratamiento. An Med Interna 24(3):138–141
Nurujjaman M et al (2009) Comparative study of nonlinear properties of EEG signals of normal persons and epileptic patients. Nonlinear Biomed Phys 3(6):1–5
Okazaki R et al (2015) Changes in EEG complexity with electroconvulsive therapy in a patient with autism spectrum disorders: a multiscale entropy approach. Front Human Neurosci 9:1–7
Ouyang G, Li J, Liu X, Li X (2013) Dynamic characteristics of absence EEG recordings with multiscale permutation entropy analysis. Epilepsy Res 104(3):246–252
Pijn J et al (1997) Nonlinear dynamics of epileptic seizures on basis of intracranial EEG recordings. Brain Topogr 9(4):249–270
Pincus SM (1991) Approximate entropy as a measure of system complexity. Proc Nat Acad Sci USA 88(1):2297–2301
Rajeev S, Ram Bilas P, Rajendra A (2015) An integrated index for the identification of focal electroencephalogram signals using discrete wavelet transform and entropy measures. Entropy 17(2015):5218–5240
Rating D (2014) Journal club, Wie konstant ist die EEG-Befundung?. Z Epileptologie, Springer Med 27(2):139–142
Reinicke C, Muthuraman M, Anwar AR, Mideska K, Siniatchkin M, Stephani U et al (2014) Neuronale Netzwerke bei einem Patienten mit früh kindlicher epileptischer Enzephalopathie. Klin Neurophysiol 45:179–182
Richman J, Moorman J (2000) Physiological time series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278(H2039):H2039–H2049
Schmitt B, Wohlrab G (2013) EEG in der neuropädiatrie. Springer Medizin. Springer, Berlin
Shannon CE (1948a) A mathematical theory of communication. Bell Syst Tech J 27(1948):379–423
Shannon CE (1948b) A mathematical theory of communication. Bell Syst Tech J 27(1948):623–656
Shorvon D (2011) The etiologic classification of epilepsy. Epilepsy 52(6):1052–1057
De Wu S et al (2013) Time series analysis using composite multiscale entropy. Entropy 2013(15):1069–1084
Stam C (2003) Chaos, Continuous EEG, and cognitive mechanisms: a future for clinical neurophysiology. Am J END. Technol 43:211–227
Stam C, Van Straaten E (2013) Structure out of chaos: functional brain network analysis with EEG, MEG, and functional MRI. Eur Neuropsychopharmacol 23(1):7–18
Stam C, van Woerkom T, Keunen R (1997) Non-linear analysis of the electroencephalogram in Creutzfeldt-Jakob disease. Biol Cybern 77(4):247–256
Steinhoff B, Bast T (2013) Compendium antiepileptic drugs. German Society of Epileptology (Deutsche Gesellschaft für Epileptology e. V.), Berlin, Germany, pp 2012–2013
Stephani U (2006) The natural history of myoclonic astatic epilepsy (Doose Syndrome) and Lennox Gastaut Syndrome. Epilepsia 47(2):53–55
Tsuchida T et al (2016) American clinical neurophysiology society: EEG guidelines introduction. J Clin Neurophysiol 4(33): 301–302
WHO (2013a) WHO model list of essential medicines. 18th List, 2013. WHO, World Health Organization, Geneive, Italy
WHO (2013b) WHO model list of essential medicines for children. 4th List, 2013. WHO, World Health Organization, Geneive, Italy
Pan Y-H et al (2010) Efficient computation of multiscale entropy in biomedicine. In: International symposium on computer, communication, control and automation, vol 2
Zavala-Yoé R, Ramírez-Mendoza (2016) EEG long-term dynamics to measure progress of concurrent patients in drug-resistant childhood syndromes. In: Kalinin VV (ed) Epileptology - the modern state of science, chap 1. InTech, Janeza Trdine 9, 51000 Rijeka, Croatia, pp 3–25
Zavala-Yoé R, Ramírez-Mendoza R, Cordero LM (2015a) Entropy measures to study long term simultaneous evolution of children in Doose and Lennox-Gastaut Syndromes. J Integr Neurosci 15(2):205–223
Zavala-Yoé R, Ramírez-Mendoza R, Cordero LM (2015b) Novel way to investigate evolution of children refractory epilepsy by complexity metrics in massive information. Springer Plus 4(437):1–33
Zavala-Yoé R, Ramirez-Mendoza R, Jimenez-Botello LC (2015c) Mathematical complexity as alternative to deal with multiple massive data eeg in children epilepsy. Z Epileptologie, Springer Medizin 28(1):12
Acknowledgements
Our gratitude to Prof. Natasha Maurits and Prof. O. Brouwer from the University Medical Center Groningen (UMCG), The Netherlands for having sent us a database which was very useful for this work. On the other hand, authors did not have any funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Rights and permissions
About this article
Cite this article
Zavala-Yoe, R., Ramirez-Mendoza, R.A. Dynamic complexity measures and entropy paths for modelling and comparison of evolution of patients with drug resistant epileptic encephalopathy syndromes (DREES). Metab Brain Dis 32, 1553–1569 (2017). https://doi.org/10.1007/s11011-017-0036-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0036-y