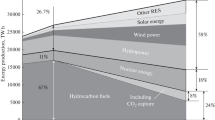
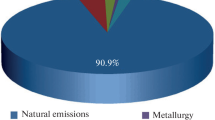
We consider actual problems of production, transportation, and storage of hydrogen and demonstrate the urgency and prospects of subsequent investigations and introduction of hydrogen-based technologies in powder metallurgy. We present predictions of an international organization according to which commercial mass production of hydrogen is expected in the nearest future. This will make hydrogen less costly and more available for the extensive applications in various branches of science and industry. It is indicated that hydrogen is not only a promising alternative energy carrier but also an efficient reducer for numerous metals and alloys and an element applied in various technologies of powder metallurgy aimed at getting broad ranges of high-quality products. It is assumed that hydrogen can find extensive applications in industry in the future.


Similar content being viewed by others
References
J. Tang, M. Chu, F. Li, C. Feng, Z. Liu, and Y. Zhou, “Development and progress on hydrogen metallurgy,” Int. J. Miner., Met. Mater., 27, No. 6, 713–723 (2020); https://doi.org/10.1007/s12613-020-2021-4.
M. Dreillard, P. Broutin, P. Briot, T. Huard, and A. Lettat, “Application of the DMX TM CO2 capture process in steel industry,” Energy Procedia, 114, 2573–2589 (2020); https://doi.org/10.1016/j.egypro.2017.03.1415.
P. Prathap and D. Senthilkumaran, “Reduction of environmental impact by incorporating performance oriented life cycle assessment,” Environ. Protect. Eng., 42, No. 1, 113–122 (2016).
T. Eglinton, J. Hinkley, A. Beath, and M. Dell’Amico, “Potential applications of concentrated solar thermal technologies in the Australian minerals processing and extractive metallurgical industry,” J. Miner. Met. Mater. Soc., 65, No. 12, 1710–1720 (2013); https://doi.org/10.1007/s11837-013-0707-z.
N. R. Neelameggham, “Soda fuel cycle metallurgy — choices for CO2 reduction,” in: N. R. Neelameggham and R. G. Reddy (editors), Carbon Dioxide Reduction Metallurgy, Metals and Materials Society (Tms) (2008), pp. 135–146.
Q. Bellouard, S. Rodat, M. Grateau, and S. Abanades, “Solar biomass gasification combined with iron oxide reduction for syngas production and green iron metallurgy,” Front. Energy Res., 8, 1–11 (2020); https://doi.org/10.3389/fenrg.2020.00066.
S. Tonomura, N. Kikuchi, N. Ishiwata, S. Tomisaki, and Y. Tomita, “Concept and current state of CO2 ultimate reduction in the steelmaking process (COURSE50) aimed at sustainability in the Japanese steel industry,” J. Sustain. Metall., 2, No. 3, 191–199 (2016); https://doi.org/10.1007/s40831-016-0066-4.
A. Babich, D. Senk, and S. Born, “Interaction between co-injected substances with pulverized coal into the blast furnace,” ISIJ Int., 54, No. 12, 2704–2712 (2014); https://doi.org/10.2355/isijinternational.54.2704.
C. Pichler and J. Antrekowitsch, “Pyrolysis gas as a renewable reducing agent for the recycling of zinc- and lead-bearing residues: A status report,” J. Miner. Met. Mater. Soc., 69, No. 6, 999–1006 (2017); https://doi.org/10.1007/s11837-017-2341-7.
T. Griessacher and J. Antrekowitsch, “Utilization of biomass at the recycling of heavy metal containing wastes,” Waste Biomass Valor., 3(3) (2012), pp. 369–374; https://doi.org/10.1007/s12649-012-9126-6.
T. Griessacher, J. Antrekowitsch, and S. Steinlechner, “Charcoal from agricultural residues as alternative reducing agent in metal recycling,” Biomass Bioenergy, 39, 139–146 (2012); https://doi.org/10.1016/j.biombioe.2011.12.043.
C. Baumgart, C. Weigelt, A. Lißner, S. Martin, C. G. Aneziris, and L. Krüger, “Processing of 17Cr7Mn6Ni TRIP steel powder by extrusion at room temperature and pressureless sintering,” Adv. Eng. Mater., 22, No. 6 (2020).
S. Luidold and H. Antrekowitsch, “Hydrogen as a reducing agent: Thermodynamic possibilities,” J. Miner. Met. Mater. Soc., 59, No. 10, 58–62 (2007); https://doi.org/10.1007/s11837-007-0133-1.
M. Kundak, L. Lazic, and J. Crnko, “CO2 emissions in the steel industry,” Metalurgija, 48, No. 3, 193–197 (2009).
Z. Chen, J. Dang, X. Hu, and H. Yan, “Reduction kinetics of hematite powder in hydrogen atmosphere at moderate temperatures,” Metals, 8, No. 10, 751 (2018); https://doi.org/10.3390/met8100751.
N. L. Solodova, R. R. Minigulov, and E. A. Emel’yanycheva, “Hydrogen as a promising energy carrier. Contemporary methods for the production of hydrogen,” Vest. Kazan. Tekhnol. Univ., No. 3 (2015); URL: https://cyberleninka.ru/article/n/vodorod-kakperspektivnyy-energonositel-sovremennye-metody-polucheniyavodoroda (Date of access: 22.07.2020).
Hydrogen Council. Hydrogen Scaling up, a Sustainable Pathway for the Global Energy Transition [Electronic Resource]; URL: https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-scaling-up-Hydrogen-Council.pdf (Date of access: 07.07.2020).
Hydrogen Council. Path to Hydrogen Competitiveness, a Cost Perspective [Electronic Resource]; URL: https:// hydrogencouncil.com/wp-content/uploads/2020/01/Path-to-Hydrogen-Competitiveness_Full-Study-1.pdf (Date of access: 09.07.2020).
V. N. Fateev, O. K. Alekseeva, S. V Korobtsev, and E. A. Seregina, “Problems of accumulation and storage of hydrogen,” Kimya Problemleri, No. 4 (16), 453–483 (2018); URL: https://cyberleninka.ru/article/n/problemy-akkumulirovaniya-ihraneniyavodoroda (Date of access: 22.07.2020).
N. Tenhumberg and K. Buker, “Ecological and economic evaluation of hydrogen production by different water electrolysis technologies,” Chem. Ing. Tech., 92, No. 2, 1–11 (2020).
X. Liu, G. Cao, Y. He, M. Yang, and Z. Liu, “Reduction of oxide scale with hydrogen,” J. Iron Steel Res. Int., 21, No. 1, 24–29 (2014); https://doi.org/10.1016/s1006-706x(14)60005-4.
Y. Mohassab, M. Elzohiery, and H. Y. Sohn, “Flash reduction of magnetite and hematite concentrates with hydrogen in a lab-scale reactor for a novel ironmaking process,” in: Proc. of the 7th Internat. Symp. on High-Temperature Metallurgical Processing (February 14–18, 2016, Downtown Nashville, Tennessee), Wiley (2016), pp. 3–10.
Z.-F. Li, Y. Gao, G.-M. Cao, and Z.-Y. Liu, “High-efficiency reduction behavior for the oxide scale formed on hot-rolled steel in a mixed atmosphere of hydrogen and argon,” J. Mater. Sci., 55, No. 4, 1826–1839 (2020).
N. M. Gaballah, A. F. Zikry, M. G. Khalifa, A. B. Farag, N. A. El-Hussiny, and M. E. H. Shalabi, “Kinetic reduction of mill scale via hydrogen,” Sci. Sinter., 46, No. 1, 107–116 (2014).
Y. Z. Lang, R. R. Arnepalli, and A. Tiwari, “A review on hydrogen production: methods, materials and nanotechnology,” J. Nanosci. Nanotechnol., 11, No. 5, 3719–3739 (2011).
E. M. Fainshmidt, V. F. Pegashkin, and L. A. Babysheva, “Raw materials for the production of sintered components in mechanical engineering,” Izv. Vyssh. Uchebn. Zaved. Mashinostr., No. 1 (2008); URL: https://cyberleninka.ru/article/n/syrie-dlya-polucheniya-spechennyhdetaley-mashinostroeniya (Date of access: 17.07.2020).
F. Akagi and Y. Ishii, “Effects of anisotropy field dispersion and grain boundary on coercivity and squareness ratio for HDDR-processed NdFeB powders,” AIP Adv., 8, No. 5, 056201 (2018).
L.-W. Cai, S. Guo, G.-F. Ding, R.-J. Chen, J. Liu, D. Lee, and A.-R. Yan, “Influence of RE-rich phase distribution in initial alloy on anisotropy of HDDR powders,” Chin. Phys. B, 24, No. 9, 097505 (2015); https://doi.org/10.1088/1674-1056/24/9/097505.
H. R. Cha, J. G. Lee, Y. K. Baek, J. H. Yu, H. W. Kwon, and Y. D. Kim, “Synthesis of ultra-fine grained Nd–Fe–B magnetic powder by the control of DR speed during HDDR process,” Korean J. Met. Mater., 51, No. 5, 371–376 (2013).
S. K. Pal, K. Güth, T. G. Woodcock, L. Schultz, and O. Gutfleisch, “Properties of isolated single crystalline and textured polycrystalline nano/sub-micrometre Nd2Fe14B particles obtained from milling of HDDR powder,” J. Phys. D: Appl. Phys., 46, No. 37, 375004–375012 (2013).
V. I. Kostikov, V. Yu. Dorofeev, and Zh. V. Eremeeva, “Protective atmospheres in powder metallurgy,” Tekhnol. Met., No. 12, 30–33 (2007).
V. I. Kostikov, V. Yu. Dorofeev, D. A. Chumak-Zhun’, A. P. Ul’yanovskii, Zh. V. Eremeeva, and D. L. Yaitskii, “Reduction of oxide films during consolidation of the charge used to make powder semifinished products,” Metallurg, No. 7, 55–57 (2008); English translation: Metallurgist, 52, 415–419 (2008).
L. Kral and J. Cermak, “Improvement of hydrogen storage properties of Mg by catalytic effect of Al-containing phases in Mg–Al–Ti–Zr–C powders,” Int. J. Hydrogen Energy, 44, No. 26, 13,561–13,568 (2019).
Y.-C. Pan, J.-X. Zou, X.-Q. Zeng, and W.-J. Ding, “Hydrogen storage properties of Mg–TiO2 composite powder prepared by arc plasma method,” Trans. Nonferr. Met. Soc. China, 24, No. 12, 3834–3839 (2014).
M. Meyer and L. Mendoza-Zelis, “Mechanically alloyed Mg–Ni–Ti and Mg–Fe–Ti powders as hydrogen storage materials,” Int. J. Hydrogen Energy, 37, No. 19, 14,864–14,869 (2012).
A. A. Kovalevskii, A. S. Strogova, V. A. Labunov, and A. A. Shevchenok, “Nano- and microstructural silicon powders in the synthesis and storage of hydrogen,” in: Z. Bartul and J. Trenor (editors), Advances in Nanotechnology, Vol. 18, Chapter 5, pp. 173–189, Nova Science (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 3, pp. 63–67, March, 2021.
Rights and permissions
About this article
Cite this article
Akhmetov, A.S., Eremeeva, J.V. Prospects for the Extensive Application of Hydrogen in Powder Metallurgy. Metallurgist 65, 314–319 (2021). https://doi.org/10.1007/s11015-021-01159-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01159-0