Abstract
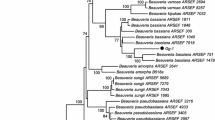
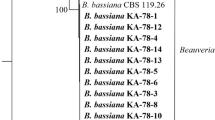
The pine processionary moth Thaumetopoea pityocampa (Den. & Schiff.) is one of the most harmful pests to pine species in Mediterranean countries including Turkey. Caterpillars of T. pityocampa are not only significantly harmful to forest trees but also responsible for various allergic reactions in humans and animals. In this study, in order to find a more effective and safe biological control agent against T. pityocampa, we investigated fungal pathogens of T. pityocampa in the Black Sea Region of Turkey and tested their pathogenicity on it. Five different fungi were isolated and identified based on their morphological and molecular characteristics including ITS and partial sequence of EF1-[alpha]. Based on these characteristics, four isolates were identified as Beauveria bassiana cf. Clade C (Rehner and Buckley in Mycologia 97:84–98, 2005) and one isolate was identified as Beauveria bassiana. Among these isolates, B. bassiana KTU-24, B. bassiana cf. Clade C KTU-66 and KTU-67 showed the highest virulence with 100% mortality within 10 days after application. B. bassiana isolate KTU-24 produced the highest mycosis value with %100. Consequently, B. bassiana KTU-24 seems to be good candidate for further investigation as a possible biological control agent against this pest.



Similar content being viewed by others
References
Masutti L, Battisti A. Thaumetopoea pityocampa (Den. & Schiff.) in Italy. Bionomics and perspectives of integrated control. J Appl Entomol. 1990;110:229–34.
Shevelev AB, Battisti A, Volynskaya AM, Novikova S, Kostina LI, Zalunin IA. Susceptibility of the pine processionary caterpillar Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) toward δ-endotoxins of Bacillus thuringiensis under laboratory conditions. Ann Appl Biol. 2001;138:255–61.
Vega ML, Vega J, Vega JM, Moneo I, Sanchez E, Miranda A. Cutaneous reactions to pine processionary caterpillar (Thaumetopoea pityocampa) in pediatric population. Pediatr Allergy Immunol. 2003;14:482–6.
Kanat M, Alma MH, Sivrikaya F. Effect of defoliation by Thaumetopoea pityocampa (Den. & Schiff.) (Lepidoptera: Thaumetopoidae) on annual diameter increment of Pinus brutia Ten. in Turkey. Ann For Sci. 2005;62:1–4.
Vega JM, Moneo I, Armentia A, Lopez-Rico R, Curiel G, Bartolome B, Fernandez A. Anaphylaxis to a pine caterpillar. Allergy. 1997;52:1244–5.
Denneulin JC, Lamy M. Effects of a chitin inhibitor, dimilin 1- (4-chlorophenyl)-3-(2, 6-difluorobenzoyl) urea, on the oenocytes and moulting in the processionary caterpillar (Thaumetopoea pityocampa Schiff.) (Lepidoptera). Ann Endocrinol (Paris). 1977;38:405–6.
Georgevits RP. Comparison of results in the control of Thaumetopoea pityocampa Schiff. with Dimilin, Decis, Bactospeine and Thuricide HP. Anakoinoseis Idrumaton Dasikon Ereun. 1979;7:7–34.
Paparatti B, Fabozzi R. A new pathogen of the pine processionary caterpillar (Thaumetopoea pityocampa Den. et Schif.), Lepidoptera: Thaumetopoeidae. Inf Fitopatol. 1988;38:45–8.
Avtzis ND. The use of Bacillus thuringiensis against Thaumetopoea pityocampa Schiff. (Lepidoptera: Thaumetopoeidae) in Greece. USDA Forest Service General Technical Report. NE-247; 1998.
Battisti A, Longo S, Tiberi R, Triggiany O. Results and perspectives in the use of Bacillus thuringiensis Berl. var. kurstaki and other pathogens against Thaumetopoea pityocampa (Den. et Schiff.) in Italy (Lep., Thaumetopoeidae). Anz Schadlingskd Pfl. 1998;71:72–6.
Triggiani O, Tarasco E. Efficacy and persistence of entomopathogenic nematodes in controlling larval populations of Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae). Biocontrol Sci Technol. 2002;12:747–52.
Er MK, Tunaz H, Gokce A. Pathogenicity of entomopathogenic fungi to Thaumetopoea pityocampa (Schiff.) (Lepidoptera: Thaumatopoeidae) larvae in laboratory conditions. J Pest Sci. 2007;80:235–9.
Ince IA, Demir I, Demirbag Z, Nalcacıoglu R. A cytoplasmic polyhedrosis virus isolated from the pine processionary caterpillar Thaumetopoea pityocampa. J Microbiol Biotechnol. 2007;17:632–7.
Ince IA, Katı H, Yılmaz H, Demir I, Demirbag Z. Isolation and identification of bacteria from Thaumetopoea pityocampa Den. and Schiff. (Lep. Thaumetopoidae) and determination of their biocontrol potential. World J Microbiol Biotechnol. 2008;24:3005–15.
Khetan S. Microbial pest control. New York: Marcel Dekker; 2001.
Lacey LA, Goettel MS. Current developments in microbial control of insect pests and prospects for the early 21st century. Entomophaga. 1995;40:3–27.
Goettel MS, Eilenberg J, Glare T. Entomopathogenic fungi and their role in regulation of insect populations. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science. Amsterdam: Elsevier; 2005. p. 361–405.
Alves SB, Pereira RM, Lopes RB, Tamai M. Use of entomopathogenic fungi in Latin America. In: Upadhyay RK, editor. Advances in microbial control of insect pests. New York: Kluwer; 2003. p. 193–211.
Feng MG, Poprawski TJ, Khachatourians GG. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Technol. 1994;4:3–34.
Glare TR. Molecular characterization in the entomopathogenic fungal genus Beauveria. Laimburg J. 2004;1:286–98.
Glare TR, Reay SD, Nelson TL, Moore R. Beauveria caledonica is a naturally occurring pathogen of forest beetles. Mycol Res. 2008;112:352–60.
Sevim A, Demir I, Höfte M, Humber RA, Demirbag Z. Isolation and characterization of entomopathogenic fungi from hazelnut-growing region of Turkey. Biocontrol. 2010;55:279–97.
Sevim A, Demir I, Tanyeli E, Demirbag Z. Screening of entomopathogenic fungi against the European spruce bark beetle, Dendroctonus micans (Coleoptera: Scolytidae). Biocontrol Sci Technol. 2010;20:3–11.
Glare TR, Inwood A. Morphological and genetic characterization of Beauveria spp. from New Zealand. Mycol Res. 1998;102:250–6.
Rehner SA, Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98.
Meyling NV, Eilenberg J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol Control. 2007;43:145–55.
Veen KH. Recherches sur la maladie due á Metarhizium anisopliae chez le criquet pèlerin. Dissertation, Wageningen University; 1968.
Vargas-Osuna E, Ledesma MJ, Aldebis HK, Santiago-Alvarez C. Pathogens and parasitoids for the control of Thaumetopoea pityocampa (D. y Schiff) (Lep. Notodontidae). Bol Sanid Veg Plagas. 1994;20:511–5.
Humber R. Fungi: identification. In: Lacey LA, editor. Manual of techniques in insect pathology. San Diego: Academic Press; 1997. p. 153–85.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. p. 315–22.
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp. 1990;41:95–8.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994;22:4673–80.
Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20.
Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9.
Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–7.
Lacey LA, Frutos R, Kaya HK, Vail P. Insect pathogens as biological control agents: do they have a future? Biol Cont. 2001;21:230–48.
Lacey LA. Microbial control of insects. In: Capinera J, editor. In encyclopedia of entomology. Dordrecht: Kluwer; 2004. p. 1401–7.
Charnley AK. Microbial pathogens and insect pest control. Lett Appl Microbiol. 1991;12:149–57.
Sezen K, Demir I, Demirbag Z. Identification and pathogenicity of entomopathogenic bacteria from common cockchafer, Melolontha melolontha (Coleoptera: Scarabaeidae). N Z J Crop Hort. 2007;35:79–85.
Torres-Barragan A, Anaya AL, Alatorre R, Toriello C. Entomopathogenic fungi from ‘El Eden’ Ecological Reserve, Quintana Roo, Mexico. Mycopathologia. 2004;158:61–71.
Devi VPS, Prasad YG, Chowdary DA, Rao LM, Balakrishnan K. Identification of virulent isolates of the entomopathogenic fungus Nomuraea rileyi (F) Samson for the management of Helicoverpa armigera and Spodoptera littura. Mycopathologia. 2003;156:365–73.
Takatsuka J. Characterization of Beauveria bassiana Isolates from Japan using intersimple sequence repeat-anchored polymerase chain reaction (ISSR-PCR) amplification. Appl Entomol Zool. 2007;42:563–71.
Acknowledgments
We would like to thank Dr. Richard Humber for his kind help with the morphological characterization of fungi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sevim, A., Demir, I. & Demirbağ, Z. Molecular Characterization and Virulence of Beauveria spp. from the Pine Processionary Moth, Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae). Mycopathologia 170, 269–277 (2010). https://doi.org/10.1007/s11046-010-9321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-010-9321-6