Abstract
It has been proposed that oligopeptides may be formed in submarine hydrothermal systems (SHSs). Oligopeptides have been synthesized previously under simulated SHS conditions which are likely geochemically implausible. We have herein investigated the oligomerization of glycine under SHS–like conditions with respect to the limitations imposed by starting amino acid concentration, heating time, and temperature. When 10−1 M glycine solutions were heated at 250°C for < 20 min glycine oligomers up to tetramers and diketopiperazine (DKP) were detectable. At 200°C, less oligomerization was noted. Peptides beyond glycylglycine (gly2) and DKP were not detected below 150°C. At 10−2 M initial glycine concentration and below, only gly2, DKP, and gly3 were detected, and then only above 200°C at < 20 min reaction time. Gly3 was undetectable at longer reaction times. The major parameters limiting peptide synthesis in SHSs appear to be concentration, time, and temperature. Given the expected low concentrations of amino acids, the long residence times and range of temperatures in SHSs, it is unlikely that SHS environments were robust sources of even simple peptides. Possible unexplored solutions to the problems presented here are also discussed.
Similar content being viewed by others
Introduction
It is generally believed that the first self-replicating systems evolved from the abiotic polymerization of environmentally supplied organic molecules (Miller and Orgel 1974; Bada 2004), although alternative models have been proposed (Morowitz et al. 2000; Huber and Wächtershäuser 2006; Shapiro 2006). Geochemically plausible polymerization reactions are thus extremely important for many origin of life scenarios.
If amino acid oligomers or polymers (polypeptides) were important for the origin of life, some combination of environmental parameters must have allowed for oligomerization. Based on what is known from physical organic chemistry this may be problematic. To form a peptide bond in water, considerable energy must be supplied (estimated as ∼ 2.5–3.6 kcal/mole (Meggy 1956; Martin 1998), which could be provided by a high temperature environment. However, simultaneously competing hydrolytic decay of the reactant amino acids (White 1984) must be avoided. There have been numerous demonstrations of the formation of oligopeptides under conditions proposed to mimic the conditions found in hydrothermal environments, however, such experiments often ignore several crucial geochemical parameters. We review here previous work on abiotic peptide synthesis in SHS environments, and present experimental data which attempt to delineate the synthetic “window” for amino acid oligomerization in aqueous solution.
Amino acids can be oligomerized by heating in the dry state at 150°–180°C (Fox and Harada 1958), or in concentrated aqueous solution (9–18 M glycine anhydride) when heated at high temperature (180°C) for short periods of time (3–68 h) (Meggy 1953). Heating concentrated aqueous glycinamide (2 M final concentration at 80° C (Yanagawa et al. 1984) or glycinamide (18 M) and ammonia (2–8 M) at 100°C also produces peptides (Oró and Guidry 1960). Amino acids can be robustly polymerized in cooler, more dilute solutions when activating agents are added (Flores and Leckie 1973; Liu and Orgel 1998a, b; Maurel and Orgel 2000; Leman et al. 2004). Plausibly prebiotic reactions that model the conditions found in alternating dry/wet beach or lagoon environments have also been demonstrated to give small yields of short oligopeptides (Rode et al. 1999; Taillades et al. 1999; Bertrand et al. 2001).
Since direct condensation of amino acids to peptides in solution is not a thermodynamically favorable process (Flegmann and Tattersall 1979), most authors have chosen to work with high initial concentrations of amino acids (1–10−2 M) (Imai et al. 1999a, b; Tsukahara et al. 2002; Islam et al. 2003), activated amino acids (Liu and Orgel 1998a, b), or potential prebiotic condensing agents (Flores and Leckie, 1973; Leman et al. 2004) that allow facile synthesis of peptides. In addition, to avoid the problem of rapid amino acid degradation at high temperatures, most authors have worked with relatively short heating times. High temperatures are generally unnecessary when condensing agents are used, but these types of simulations side-step the issue that low concentrations and high temperatures are incompatible with oligopeptide formation. The range and nature of conditions chosen in previous experimental work in effect define several of the parameters which limit amino acid oligomerization. The exploration of the conditions where such reactions fail is useful for delineating plausible environments for abiotic oligopeptide synthesis.
It has been proposed that hydrothermal vent systems may have been important for prebiotic peptide synthesis (Huber and Wächtershäuser 1998; Imai et al. 1999a, b; Tsukahara et al. 2002, Shock 1992c). Simulations have examined the effects of high temperatures on amino acid oligomerization, sometimes in the presence of possible catalysts such as metal ions, fatty acids or clays. Most experiments demonstrating hydrothermal peptide synthesis start with extremely high and geochemically implausible concentrations of amino acids, which ignores their original source, heated for very short periods of time at high temperatures, which neglects the characteristics of the environments where these reactions are proposed to occur. For example, Yanagawa and Kojima (1985) showed that when a 0.8 M total concentration mixture of amino acids was heated between 250° and 350°C for 6 h, microspheres presumably composed of polypeptides were formed. Imai et al. (1999a) obtained oligomers up to the hexamer from concentrated glycine solutions (10−1 M) exposed to high temperatures (200°–250°C) and pressures (240 Bar) for short (34 or 78 s) heating times. Ogata et al. (2000) showed that alanine and glycine could be co-polymerized to form short oligomers under similar conditions of temperature, pressure, initial monomer concentration and heating time. Although oligomerization occurs under these reaction conditions, the simulation conditions are not geochemically plausible. Much lower concentrations of abiologically synthesized amino acids are likely to be present in hydrothermal environments (Shock 1992a; Islam et al. 2003), as discussed below. Natural hydrothermal fluids also typically experience markedly longer heating periods of years to decades or longer (Turekian and Cochran 1986; Shock 1992a, b; Coumou et al. 2008).
Amino acids are the minimum requirement to form peptides. There has been considerable debate as to whether amino acids are principally synthesized (Hennet et al. 1992; Marshall 1994; Islam et al. 2003) or degraded (White 1984; Bada and Miller 1988; Bada et al. 1995) in hydrothermal environments. Central to this question is whether amino acids achieve “metastable equilibrium” states during the long passage of hydrothermal fluids through submarine hydrothermal environments. This depends upon the composition of the source fluid and dissolved gases, its redox equilibration with surrounding mineral phases, and whether equilibrium, and what that equilibrium is, is attained with respect to these factors.
Abiotically synthesized amino acids may either be generated in situ in the vents, or be provided by the input of amino acids naturally present in seawater. In the former case, there must be mechanisms for hydrothermal amino acid synthesis that compete with thermal degradation. The “equilibrium” attained would depend on the raw materials provided for synthesis, the availability of catalysts, and the physical conditions such as the pH, temperature, salinity and mineralogy found in such environments. In the latter case, amino acids could be derived from solution phase bulk ocean chemistry such as the Strecker synthesis from atmospheric or extraterrestrial input of precursor materials such as HCN, NH3 and aldehydes or ketones (Stribling and Miller 1987), or via direct input of amino acids from meteorites or interplanetary dust particles (Chyba and Sagan 1992). The steady state concentrations in bulk seawater would depend on the balance between input, synthesis and degradation, which depend on the physical parameters of the ocean environment (pH, temperature, UV flux, mixing rate, etc.).
Literature estimates of plausible prebiotic oceanic amino acid concentrations can be used to obtain a range of plausible amino acid concentrations in submarine hydrothermal systems. These estimates can then be compared with laboratory investigations of the mechanisms and kinetics of hydrothermal abiotic peptide formation to assess the potential for amino acid oligomerization in SHSs.
If all of the nitrogen in the Earth’s crustal, atmospheric, and ocean reservoirs were present as amino acids dissolved in oceans of the present volume the concentration would be ∼ 0.2 M (Schwartz 1981). This would be the absolute highest possible amino acid concentration which could have been obtained in the primitive oceans. It is unlikely, however, that all of the nitrogen on Earth’s surface could have been completely converted into amino acids or other water soluble nitrogen species, including ammonia, nitrite or nitrate. Lower amino acid concentrations of ∼ 3 × 10−4 M have been estimated based on favorable atmospheric synthesis from fairly reducing atmospheres (Stribling and Miller 1987). The existence of an early reducing atmosphere is presently contentious, suggesting that even these estimates may be somewhat high.
For the lower limit, a maximum meta-stable equilibrium of ∼ 10−9 M for glycine in HT vent environments has been estimated by Shock (1992a), although it was also estimated that this value could be up to 10 orders of magnitude lower. Thus, the relevant amino acid concentrations for oligomerization studies in hydrothermal systems probably lie somewhere between 3 × 10−4 M and 10−19 M.
We have herein studied the oligomerization of amino acids in SHS environments with respect to concentration, heating time and temperature. The potential effects of variables such as the presence of metals and mineral surfaces, pH and pressure are also discussed.
Materials and Methods
Glycine, oligoglycines (gly2-gly7), and 2, 4-diketopiperazine were purchased from Aldrich. Solutions were prepared by dissolving glycine in 500 mL of doubly distilled H2O in a pre-sterilized pyrex bottle, and passing this solution through a 15 mL stainless alloy (Hastelloy C-22) flow reactor heated between 50° C and 300° C (Fig. 1). The reactor, similar to the one used by Imai et al. (1999a), is described in detail elsewhere (Aubrey et al. 2008). Effluent was passed through a coil immersed in a 4°C cooling bath to mimic the entry of the vent fluid into cooler surrounding ocean water. The temperature of the solution in the heated stainless column was controlled using a jacket-heating element with two type-K thermocouples wired in parallel set deep within the reactor wall. Solutions were allowed to reach the set temperature at the desired flowrate, and then sufficient time (>1 residence time) was allowed to elapse before fraction collection to ensure that the liquid which had passed through the reactor had been exposed to the appropriate temperature before collection. By varying the flow rate of the fluid through the chamber, the exposure time of the fluid could be varied to test the effect of residence time on oligomerization.
Reactor composition has been shown to have potentially significant effects on the rates of amino acid decarboxylation. For example, decarboxylation rates in quartz (Li and Brill 2003a) and 316 stainless steel (Sato et al. 2002) reactors are similar, while decarboxylation in Au-Ti alloys reactors is significantly slower (Qian et al. 1993). Hastelloy C-22 was chosen based on reactor designs utilized in previous studies (Imai et al. 1999a) and the fact that it shows superior resistance to corrosion at high temperatures under both acidic and alkaline conditions.
Hydrothermally-exposed solutions were analyzed for amino acids and amine degradation products according to the method of Zhao and Bada (1995) after derivatization with o-phthaldialdehyde N-acetyl-L-cysteine. The solutions were also assayed for UV absorbance using an HP8452A diode array detector using 1 cm path length quartz cuvettes, and by RP-HPLC with UV absorbance detection at 260 nm using a Phenomenex 150 × 4.6 mm C18(2) column and 0.1 M pH 5 ammonium acetate buffer as eluent. Oligomeric products were analyzed according to the procedure of Imai et al. (1999a) using RP-HPLC with UV detection at 210 nm with a Phenomenex 150 × 4.6 mm C-18(2) column eluted with pH 2.5 50 mM KH2PO4 containing 7.2 mM C6H13SO3Na at a flow rate of 0.5 mL min−1.
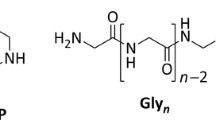
Glycine oligomers (gly1-gly7; glyN = glycine N-mer) were measured, as well as the cyclic dipeptide, diketopiperazine (DKP). The higher molecular weight peptides (gly4-gly7) are expected to form in much lower abundances than the shorter oligomers (gly2-gly3). However, the detection limits for higher length oligomers are lower because the UV extinction coefficient at 210 nm increases with chain length. Some error may arise from the fact that the solubility of oligomers decreases with chain length, however, it is unlikely that the amount of oligomer produced in any case was above the aqueous solubility limit at room temperature.
Results
In our experiments, 10−1 M initial glycine solutions produced a straw colored effluent at high temperature, which produced a precipitate upon cooling. Redissolution and analysis of this material showed that it did not consist of glycine oligomers. Heating 10−1 M initial glycine solutions produced oligomers up to the tetramer after 20 min at 250°C and 2400 psi (Fig. 2a) in agreement with previous experiments (Imai et al. 1999a, b).
Log oligopeptide yields or glycine recoveries vs. temperature for hydrothermal experiments run at 2400 psi (∼165 bar) for 20 min with initial glycine concentrations of (A) 10−1 M, (B) 10−2 M, and (C) 10−3 M. Log % yield = log [(peptide yield/theoretical peptide yield) × 100]; Log % recovery = log [(glycine detected/glycine initial) × 100]. Glycine was the only product detected in the lowest concentration experiment (C). The scale is changed to shown percent input monomer recovery [(glycine detected/glycine initial) × 100] to enhance the observation that monomer degradation becomes more significant at high temperature and low initial monomer concentration. Error bars reported for glycine correspond to a 10% measurement error. ♦ = glycine; ○= DKP; ● = gly2; ▴ = gly3; ▪ = gly4
At temperatures below 150°C no elongation products were detectable from 10−1 M initial concentration of glycine after 20 min. Total recovery of starting material in the form of glycine and peptides was ∼ 99.9%, 99.1%, 73.9% and 61.1% from 100°C, 150°C, 200°C and 250°C, respectively, after 20 min. The discrepancy between the mass balance of the input glycine and recovered products was easily accounted for by the detection of large amounts of methylamine in the 200°C and 250°C reactions, indicating that thermal decarboxylation of glycine was the dominant degradative reaction under these conditions. When these reactions were repeated using 10−2 M initial glycine, no condensation products above gly2 and DKP were formed after 20 min between 100°C and 250°C (Fig. 2b). Using 10−3 M initial glycine no dimers (Gly2 or DKP) above the detection limit of ∼ 0.002% yield (10−6 M) were detected after 20 min at any temperature, while the rates of decomposition of glycine were approximately those reported in Li and Brill (2003a) (Fig. 2c), suggesting that control of oxygen fugacity was not a significant variable in these reactions.
In experiments where oligopeptides were detected, heating time was found to be a crucial variable. Although oligopeptides up to the hexamer were detected after 15 min of heating using 10−2 M initial glycine at 200°C, after 20 min no peptides longer than trimers were detectable, and after 30 min, no peptides longer than dimers were detectable (Fig. 3).
Peptide yield and glycine recovery versus time at 2400 psi (165 bar) at 200°C from 10–2 M initial glycine. Log % yield = log [(peptide yield/theoretical peptide yield) × 100]; Log % recovery = log [(glycine detected/glycine initial) × 100]. ♦= glycine; ○= DKP; ●= gly2; ▲= gly3; ▪= gly4, ▲= gly5,▪=gly6. Yields are reported based on input nitrogen (%N). The trendlines for gly, DKP, gly2, and gly3 are intended only as guides and assume initial product yields at time zero of <0.03% (just above the detection limit) and >1st order kinetics. Peptides >gly3 were only detectable at 15 min of exposure at 200°C
Discussion
Glycine was chosen to investigate amino acid oligomerization because it is the simplest amino acid and the complications of stereochemistry and thermal racemization can be ignored because it is achiral. Using a single amino acid type also simplifies analysis of the polymerization products, though the formation of the cyclic dipeptide of glycine (Steinberg and Bada 1981), diketopiperazine (DKP), was also investigated. As glycine is one of the more stable α-amino acids (Li et al 2002; Li and Brill 2003a, 2003c), the conclusions reached here are likely to be largely applicable to most α-amino acids, as well as β- and γ-amino acids.
It is difficult to experimentally simulate the effect of geochemically relevant heating times on the chemistry of hydrothermal fluids. It has been estimated that fluids in on-axis hydrothermal vents experience high temperature (350°C) heating for several years (Kadko and Butterfield 1998) to decades (Turekian and Cochran 1986), although many intermediate and variable temperature regimes also exist. Residence times in lower temperature off-axis hydrothermal circulation regimes are often on the order of thousands of years (Elderfield and Schultz 1996). Experiments conducted over the course of hours to days may thus seriously underestimate the importance of degradation reactions on longer time-scales, and thus overestimate the plausibility of polymerization in SHSs.
Oligomerization is a concentration dependent 2nd-order reaction, while peptide hydrolysis and amino acid degradation are pseudo-1st order reactions, and are largely concentration independent. Peptide elongation depends on the ratios of the forward and reverse reactions, and is therefore highly sensitive to monomer concentration. Thus, the starting and possible equilibrium concentrations of amino acids and peptides are extremely important for evaluating the possibility of peptide oligomerization, regardless of the overall thermodynamic favorability of the reactions.
These data suggest that at extended reaction times the degradation of monomers draws the reaction away from polymerization. Methylamine, one of the principal thermal decomposition products of glycine (Snider and Wolfenden 2000) was detectable in all of these reactions and accounted for the mass balance of glycine loss. Interestingly, glycine methylamide, the condensation product of methylamine and glycine, was not detected in any reaction.
Glycine oligomerization in these reactions most likely occurs via two principal mechanisms: 1) the direct condensation of free amino groups and free carboxyl groups in the ionized or unionized states (Martin 1998); or 2) via condensation of DKP with the free amino group of an amino acid or oligopeptide (Nagayama et al 1990). The reverse reactions likely also occur. DKPs are formed in high yield by heating concentrated solutions of amino acids (Meggy 1953). Reaction of amino acids with DKP has been shown to produce short peptides (Nagayama et al. 1990). Proteins non-enzymatically decompose more rapidly via the loss of 2 amino acid units (dipeptides or DKPs) from their N-termini, resulting in the formation of an n-2 polypeptide and a DKP molecule (Steinberg and Bada 1981; Steinberg and Bada 1983; Gaines and Bada 1988), than by internal peptide bond hydrolysis (Radzicka and Wolfenden 1996). These two observations suggest a significant contribution of a reversible peptide elongation and degradation mechanism involving DKPs. A plausible reaction scheme which takes into account previously observed chemistry and the detected products in our experiments is presented in Fig. 4.
It has been suggested that much of the potentially important synthetic organic chemistry which may occur in primitive hydrothermal environments depends on the “freezing out” of metastable equilibrium chemistry which occurs under the regimes encountered in hydrothermal systems when they are suddenly injected into the lower temperature, more oxidizing bulk ocean (Shock et al 2000). As an experimental demonstration of this idea Imai et al. (1999a) showed that concentrated glycine solutions (10−1 M) exposed to high temperatures (200° – 250°C) and pressures (24.0 MPa) produce oligomers in a flow reactor system using 1.0 to 1.3 h residence times when the solutions were quenched in a cold bath after exposure to high temperature and pressure. Apparent “steady-state” concentrations of oligomers were thus attained. This was interpreted to mean that a quenched high-temperature thermodynamic equilibrium was reached in the system. However, high-temperature cycling times of 30 and 78 s were used, so the actual exposure to heat was much less than 1.0–1.3 h due to the amount of exposure at much lower temperatures during the quenching stage, thus these results are in agreement with those found here. The experiments reported here also include a rapid quenching stage, thus, whatever advantages are to be gained by quenching must be due to as yet unexplained phenomena which are not addressed herein.
Assuming that residence times in SHSs are sufficiently long for amino acids to be equilibrated with their decomposition products and ambient fluid composition to reach a “metastable equilibrium”, the equilibrium concentrations of reactants and products can be estimated. Such equilibria would depend on the mantle and oceanic source water composition, the “metastable” amino acid concentration, and the amount of H2 produced by basalt weathering in the vents, which could potentially affect the stability of reduced species such as amino acids (although this seems unlikely to be the case, see above), among other considerations.
It is inaccurate to speak of equilibrium in these systems, as some species may not reach equilibrium on the timescales investigated in laboratory experiments. For example, in the case of the decarboxylation of glycine, which produces methylamine and CO2, the equilibrium between CO2 and methylamine is unknown (although it can be estimated from the free energy of the reaction) but presumably lies very far towards decarboxylation at most geochemically reasonable concentrations of CO2 and methylamine, and most conditions of temperature and pressure, provided there is a kinetically accessible pathway for these species’ interconversion. Such a pathway should exist according to the principle of microscopic reversibility, but more complicated interconversion processes may also exist. The relevant reactions for aqueous glycine, including reversible formation and degradation reactions, can be simplified as:
The overall equilibrium equation becomes:
Assuming a mechanistic pathway exists for the conversion of H2, CO2, and N2 to glycine in hydrothermal vent environments, Shock (1992a) estimated a maximal “meta-stable” equilibrium concentration of ∼ 10−9 M from 1 bar of N2 and 10 bars of CO2, which is ∼ 7 orders of magnitude below the minimum starting concentration of glycine found here to produce even dipeptides.
These concentrations must be viewed in light of the likely evolution of the primitive Earth. It is believed that the early Earth’s atmosphere accumulated via out-gassing of the material from which the Earth formed. It is also widely believed that the majority of the Earth’s nitrogen pool was already present in the early atmosphere in the form of N2 from very early on, and has not been greatly recycled via hydrothermal circulation due to the low solubility of N2 in ocean water (Zhang and Zindler 1993). Thus the activity of N species was probably many orders of magnitude less than 1 bar in primitive hydrothermal systems once stable oceans were present, except perhaps very early during Earth’s evolution. Likewise, while 10 bars of CO2 are required to keep the early Earth’s surface temperature warm enough to be consistent with evidence for liquid water in the geological record via solar flux models (Kasting and Ackerman 1986), this would not mean that there were 10 bars of CO2 in hydrothermal systems. Rather, the concentration of C species would have been considerably lower (Zhang and Zindler 1993).
Using the free energy of hydrolysis of a dipeptide of ∼ 3.6 kcal/mole (Meggy 1956; Martin 1998), equilibria for amino acid dimerization can be estimated between 10−2 and 5 × 10−2 M−1 between 100°C and 300°C. Using the free energy of hydrolysis of a tripeptide of ∼ 2.5 kcal/mole (Martin 1998), equilibria for amino acid elongation can be estimated between 2 and 0.13 M−1 between 100°C and 300°C, assuming there is no degradation of monomer during the approach to equilibrium. Using the high Keq values, and the high-end amino acid concentrations estimated above (3 × 10−4 M to 10−9 M), the equilibrium concentrations of oligomers are calculated in Table 1 below.
A concentration of ∼10−45 M is equivalent to a single molecule in the entire modern ocean volume (1021 l). For example, a 10−9 M initial glycine concentration at 300°C, the approximate temperature of a black smoker hydrothermal vent effluent, would produce a pentamer concentration of 4 × 10−46 M, or less than 1 pentamer molecule per entire ocean volume. For the high-end glycine upper-limit concentration estimate (3 × 10−4 M), there would be approximately one 10-mer molecule per entire ocean volume at 300°C. It should be noted, however, that the rapid heating of bulk seawater at low temperature to 300°C, followed by rapid low temperature quenching may only occur on very small scales globally.
For libraries containing a few amino acids, perhaps 7 simple racemic amino acids plus glycine, the sequence space is fairly well explored by such syntheses at high and low temperatures for the short (2–5 monomer unit) peptides synthesized. However, it may be questionable whether there is sufficient chemical diversity in a small library of peptides 2–5 residues long to represent a significant selection pool for catalysis, especially when these are very dilute (<10-20 M).
Thermal elongation of unactivated amino acids in aqueous solution does not seem a promising prebiotic source of oligopeptides. It is possible that high temperature polymerization might provide short oligopeptides that could help nucleate polymerization in evaporating basins, since it is energetically more favorable to elongate longer peptides than shorter ones (Martin 1998) and peptides are more stable at lower temperatures. Peptide concentration and elongation mechanisms might be more favorable in such environments.
The assumption is generally made in polymer kinetics that monomers are stable to polymerization processes, which may not be true of amino acid oligomerization in SHSs, as amino acids decompose rapidly at high temperatures. The above equilibrium calculations ignore the complication of monomer degradation over long timescales or at high temperatures. We could alternatively attempt to model these experimental results kinetically, assuming the rate constants of the parallel reactions involved were accurately known across a wide range of pH, ionic strength, and temperature conditions. Unfortunately, such calculations would be highly speculative at present as many of the crucial kinetic data are unavailable. Nevertheless, experimental data do not suggest that such an approach is likely to be profitable.
The formation of a peptide bond from two oligopeptides is considerably more favorable than the formation of a dipeptide, due to the effects of charge separation with increasing peptide length (Flegmann and Tattersall 1979; Martin 1998). Glycine dimerization is rapid in concentrated solution at high temperatures, as is the formation of DKP. DKP likely reacts with glycine and gly2 to produce higher polymers via ring opening polymerization (Fig. 4). DKP decomposes more slowly than do glycine oligomers or glycine itself, and thus DKP and gly2 persist during the reactions (Fig. 3). At lower concentrations, little dimerization occurs and DKP concentrations do not become sufficiently high to allow for oligomerization (Fig. 2b, Fig. 2c). Dimerization, cyclization and elongation equilibria are all lower at lower temperatures, however glycine is considerably more stable at lower temperatures. Monomer/oligomer equilibrium is considerably shifted to the left at lower temperatures, thus the low yields of oligomers at lower temperatures in these experiments are most likely a reflection of low equilibria rather than sluggish reaction and insufficient sampling time. The low degree of conversion of monomers seen here is likely due to slow kinetic conversion rather than poor governance of oxygen fugacity which might inhibit monomer degradation.
As glycine concentrations decrease over time due to decarboxylation (Fig. 2c), which proceeds faster at higher temperatures and is essentially irreversible, oligomerization reactions decrease in frequency and equilibrium is shifted away from polymerization. Chain growth is likely principally mediated by the ring opening polymerization of DKP, with step chain growth a minor contributor, although it has been suggested that step elongation becomes more facile at higher temperature and greater chain length due to the decreasing difference between the amino and carboxyl group pKa’s resulting in a lower activation energy barrier to peptide bond formation (Martin 1998). However, the decreasing concentration of glycine vs. time at higher temperatures makes dimerization less favorable. Oligomerization depends on high monomer concentrations for the initial formation of gly2 and thus DKP. If the kinetic model is simplified as a step polymerization of single amino acid units, then the average chain length (Nav) simplifies to the expression \(N_{av} = \left( {\frac{{k_1 }}{{k_{ - 1} }}} \right)^{1/2} \) (Orgel 1998), and the need for high monomer concentrations is readily apparent.
These results highlight the impact of residence time on oligomerization. While rapidly heating high concentrations of amino acids and quickly quenching the products at lower temperatures gives good yields of oligopeptides, if the solutions are continuously heated for long periods of time, the starting monomer degrades into simpler compounds (in the case of glycine methylamine and CO2, or to a lesser extent ammonia and glycolic acid, and then in both cases to yet simpler molecules, see Equations 1, 2, 3 and 4 above) to such an extent that the polymer-monomer equilibrium is shifted towards decomposition and depolymerization (Kozai et al. 1975). The concentration of amino acids in vent fluids and the length of time fluids experience temperatures sufficiently high to induce polymerization are likely the two most important parameters in oligomerization. If experiments are run long enough, eventually most of the starting material would be degraded from exposure to high temperatures, and the degree of polymerization would be considerably less. Previous reports need to be reexamined in this light.
The effects of pressure were not specifically investigated here. These experiments were carried out at constant pressure of 2400 psi (∼165 bar), pressures typical of many SHSs, and an ocean depth of ∼ 1500 m. As there is a good correspondence between the values measured here and literature rate constants, pressure is unlikely to have a major effect on the conclusions drawn herein. Li and Brill (2003b) showed that increased pressure has a significant positive effect on the cyclization and hydrolysis rates of dipeptides, but little effect on the decarboxylation of amino acids (Li and Brill 2003a). Thus tail-biting degradation and dipeptide hydrolysis would be enhanced at higher pressures, while monomer degradation would be relatively unaffected.
Our experiments clearly do not mimic all of the possible variables likely to be encountered in SHS’s. For example, we did not study here the potential effects of ionic strength, sulfide concentration, pH or Eh. However, reasonable predictions may be made regarding the effects of these variables, which admittedly remain to be explored. We would be surprised, though, if any if these variables markedly alter our conclusions. For example, it is unlikely that natural systems could have an ionic strength much lower than the system we have investigated here. Ionic strength has been found to have no effect on peptide hydrolysis between values of 0.5 and 2 between pH values of 4.7 and 6.8 (Radzicka and Wolfenden 1996).
Sulfide is a highly variable component of modern hydrothermal fluids (Kadko and Butterfield 1998). Sulfide could facilitate chain termination, though it might also serve to lower the energy barrier to peptide formation provided that thioacids are sufficiently stable at high temperature and pressure. If all of the species in HT fluids are metastably equilibrated, sulfur redox chemistry would likely not significantly alter our conclusions, based on known chemistry.
These experiments were unbuffered with respect to O2 and H2 fugacity. The O2 and H2 fugacities in SHSs may significantly influence degradation rates. For instance, degradation rates for several amino acids were found to decrease using a Pyrite-Pyrrhotite-Magnetite oxygen fugacity buffer solution (Andersson and Holm 2000). However, relatively little O2 was present in our experiments (estimated as ∼ 2.5 × 10−4 M based on the solubility of O2 in water at room temperature), and it seems unlikely that the amount present could have affected the overall reaction progress to a large degree. H2 has been shown to slow the rate of degradation of amino acids at high temperature, but the effect is relatively slight (Islam et al. 2003; Lemke 2003). Though H2 fugacity may affect amino acid monomer concentration, but does come into play in amino acid oligomerization reactions directly, it would be surprising if altering the H2 fugacity on any reasonable scale would significantly alter the conclusions reached here.
pH is likely to have the greatest effect on these conclusions. As these reactions were not controlled with respect to pH, the potential effects of pH can only be speculated upon. The direct (non-ring opening mechanism) formation of peptide bonds is suggested to occur principally between the unionized amino (−NH2) and carboxylic acid (−COOH) ends of amino acids and peptides (Martin 1998). Since the pKa values of amino acids and peptides change with temperature (e.g.: Δ pKa Gly-NH2 = −0.025/°C, Δ pKa Gly-COOH = −0.0019/°C (Perrin 1965)), the reactions described here are expected to be pH dependent, and the pH dependence should vary with temperature. Peptide formation should be fastest at the pH at which the greatest fraction of reacting end groups are in the unionized state, balanced by the pH value at which peptide hydrolysis is slowest (Dobry et al 1952). The concentration of unionized functional groups for glycine increases with increasing temperature as modeled in unbuffered aqueous solution (Fig. 5a). This same trend is likely to be true for higher oligomers. The pH vs. temperature in these solutions is also modeled (Fig. 5b), showing the effects of glycine as the principal buffering agent in our reactions. Until it is demonstrated otherwise, it is unlikely that peptides are more stable at extremes of pH at high temperature, as they are not at room temperature (Dobry et al 1952; Kahne and Still 1988). If peptide formation is more favorable at low pH, these conclusions are unlikely to be altered by more than an order of magnitude. Thus the limits of peptide formation are still likely governed by the low concentrations and high temperatures in geochemically equilibrated environments, rather than by pH.
Since the rate of peptide synthesis may depend on the total concentrations of unionized end groups \(\left( {\frac{{dA}}{{dt}} = k \cdot \left[ {H_2 NCH_2 COOH} \right]^2 + k \cdot \left[ {H_2 NCH_2 COO^ - } \right] \cdot \left[ {^ + H_3 NCH_2 COOH} \right]} \right)\), it is important to evaluate the relative concentrations of these as a function of pKa, which vary as a function of pH and temperature. The calculated product of the total concentration of unionized end groups at different pH values suggests a strong dependence on temperature (Fig. 6).
The maximal rate of dimerization is probably greatest near pH 3–5 at high temperature, although the net rate under these pH conditions would depend on the rate and equilibrium of acid catalyzed hydrolysis of amino acids and peptides at these temperatures. At lower temperatures, slightly acidic pH values appear to be most favorable. The effects of pH on oligomerization are likely to be complex. The decreasing difference of the amino and carboxyl pKa values may account for some of the favorability of peptide formation at high temperature (Flegmann and Tattersall 1979; Martin 1998). Nagayama and coworkers (1990) noted a complicated pH-rate behavior for the ring-opening reaction of gly, gly2, and gly3 with DKP. Kozai and coworkers (1975) found the percent conversion in the ammonia-catalyzed polymerization of DKP to be more favorable in both acid and base at 160°C, but the degree of polymerization was pH-independent. The rate of hydrolysis of peptide bonds at 25°C is highly pH dependent, with the minimum value near pH 7 (Kahne and Still 1988). Amino acid decarboxylation is fairly constant (within a factor of ∼ 2) with respect to pH at 320°C and 275 bar (Li and Brill 2003a).
Further study of the effects of pH on amino acid polymerization seems warranted, but the rapid degradation of amino acids and their generally low concentrations would probably still render unactivated oligomerization ineffective in SHSs.
We have not investigated the effects of metals or mineral surfaces on these reactions, however some comments may be made regarding their possible effects. Amino acids and short peptides bind metal ions such as Ni2+, Cu2+, Mg2+ and Fe2+ with high affinity (Datta and Rabin 1956; Sharma and Mathur 1965), and Cu2+ has been found to catalyze the formation of peptide bonds in aqueous solution, especially in the presence of high salt concentrations (Imai 1999a; Rode et al. 1999). Transition metal ions also catalyze the hydrolysis of peptide bonds (Cronin et al. 1971; Long et al. 1971; Brubaker and Sakkab 1974; Hay and Morris 1976). It seems that, aside from the potential oxidative damage transition metal ions might cause to short peptides and amino acids (Bada 1971), they could also serve as true catalysts for the synthesis of short peptides, meaning that they can speed up the approach to equilibrium, but do not alter it. It is thus unlikely that the presence of metals would change our general conclusions.
It has been suggested that amino acids and organic compounds in general on the primitive Earth would have been concentrated on mineral surfaces (Bernal 1951; Nissenbaum 1976; Hazen et al. 2001). Amino acids can be elongated efficiently on mineral surfaces using activating agents or activated monomers (Ferris et al. 1996; Hill et al. 1998; Liu and Orgel 1998b; Bertrand et al. 2001). Clays and other minerals have been shown to be catalysts for amino acid oligomerization (Lahav et al. 1978; Bujdak and Rode 1996; Porter et al. 1998; Meng et al. 2004) under drying conditions. An excellent review of the possible role of mineral surfaces in abiotic peptide formation has recently been published (Lambert 2008) which addresses some of these concerns. If mineral adsorption provides some protective effect, as has been suggested by Sowerby et al. (2001), amino acids and peptides might selectively concentrate and accumulate on mineral surfaces (Basiuk and Gromovoy 1996; Orgel 1998). This is difficult to address conclusively, but might be unlikely, since mineral adsorption/fluid phase equilibrium is attained over relatively short geological time scales.
There are an enormous number of possible minerals that could be considered, with varying affinities for different amino acids depending on their side chains (Zaia 2004). In general, adsorption equilibria mediated by ionic and weak interactions tend to be higher at lower temperatures (Grzegorczyk and Carta 1996; Sowerby et al. 2001; Domareva et al. 2004), thus peptides might tend to accumulate in regions where temperatures would be too low for elongation to occur, even if sufficient concentrations of monomer were available. It is also possible that since peptides would be in dynamic equilibrium between adsorbed states and solvated states, that whatever possible protective effect adsorption might provide would be rapidly offset by solution phase hydrolysis (de Duve and Miller 1991). Lahav and Chang (1976) and Flegmann and Scholefield (1978) came to the conclusion that in dilute monomer solutions, such as in the bulk ocean and most likely in hydrothermal vent fluids, there is little potential for concentration by minerals to overcome kinetic barriers to reaction.
Thermophoresis using porous minerals has been suggested as an interesting possibility for concentrating dilute organics in hydrothermal environments. The combination of porous minerals and temperature gradients between the mouths and ends of the exposed mineral pores could potentially solve the extreme dilution problem (Baaske et al 2007)). To our knowledge this idea has not yet been explored experimentally in this context.
Condensing agents might significantly alter these conclusions, but there are several caveats. As noted by Leman et al (2004) compounds such as carbonyl sulfide (COS) are not particularly abundant in volcanic emissions, however they are likely to be more abundant than amino acids themselves, in which event it is the balance between activating agent hydrolysis, activation rate, elongation and hydrolysis rates which become important. This is likely to be true for all potential prebiotic condensing agents such as cyanate (Flores and Leckie 1973), urea (Mita et al. 2005), and high-energy phosphates (Chung et al. 1971). Amino acid polymerization on (Ni,Fe)S surfaces has been shown to require high concentrations of CO and H2S to form oligomers (Huber and Wächtershäuser 1998). Using high concentrations of CO (400 mM), H2S (50 mM), and tyrosine (50 mM, or 102–1017 times the plausible amino acid concentrations estimated here) at most 3% dipeptide and 2 × 10−3% tripeptide were produced after 4 days at 100°C. These yields are approximately the same as from the heating of concentrated glycine solutions observed in this study ([gly]0 = 0.1 M, Fig. 2a), thus the contribution of these reagents, at concentrations likely reasonable for SHS fluids, is questionable. Lower temperature environments where amino acids can be concentrated by evaporation, such as in moderate temperature evaporative basins, might again be more significant global contributors to peptide formation.
Lastly, amino acids are considerably more stable than other biological monomers such as nucleosides and nucleotides (White 1984), thus these results suggest oligopeptide formation in SHSs, though difficult, would still be considerably more plausible than the hydrothermal polymerization of nucleic acid monomers.
Conclusions
We have investigated the oligomerization of glycine in simulated hydrothermal vent conditions and find that provided initial concentrations of amino acids are high and heating time is short, oligopeptides can be generated in low but significant yield. Such conditions are much more favorable in terms of concentration (by a factor of 103–1026), temperature, and heating time (by a factor of 104–107) than what occurs in modern SHS environments. It is possible that primitive SHS environments were considerably more reducing than modern SHS environments, though this would also have consequences for the primitive atmosphere. It is questionable whether amino acid concentrations in SHSs could ever have been high enough to allow for oligomerization to occur significantly, even if these systems were more reducing early in Earth’s history. While the monomer/polymer equilibrium is more favorable at high temperatures, monomer decomposition rapidly draws the reaction back towards net depolymerization at high temperatures. The long exposure times hydrothermal fluids typically experience would largely decompose most peptides over extremely short geological time scales. Although much remains to be investigated in greater detail, it seems unlikely that minerals or metals could have had a significant impact on this chemistry, but they may serve as true catalysts. Other, cooler environments such as evaporative basins are more likely to be suitable candidates for the concentration, polymerization, and preservation of organic molecules on primitive planets.
References
Andersson E, Holm NG (2000) The stability of some selected amino acids under attempted redox constrained hydrothermal conditions. Origins of Life Evol Biosph 30:9–23
Aubrey AD, Cleaves HJ, Bada JL (2008) The role of submarine hydrothermal systems in the synthesis of amino acids. Orig Life Evol Biosph doi:10.1007/s11084-008-9153-2
Baaske P, Weinert FM, Duhr S, Lemke KH, Russell MJ, Braun D (2007) Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc Natl Acad Sci USA 104:9346–51
Bada JL (2004) How life began on Earth: a status report. Earth Planet Sci Letts 226:1–15
Bada JL (1971) Kinetics of the nonbiological decomposition and racemization of amino acids in natural waters. Adv Chem Ser 106:309–331
Bada JL, Miller SL (1988) Submarine hot springs and the origin of life. Nature 334:609–611
Bada JL, Miller SL, Zhao M (1995) The stability of amino acids at submarine hydrothermal vent temperatures. Origins of Life Evol Biosph 25:111–18
Basiuk VA, Gromovoy TY (1996) Estimation of thermodynamic parameters for amino acid adsorption on silica from water using high-performance liquid-chromatographic technique. Pol J Chem 70:476–82
Bernal JD (1951) The Physical Basis of Life. Routledge & Kegan Paul, London
Bertrand M, Bure C, Fleury F, Brack A (2001) Prebiotic polymerisation of amino acid thioesters on mineral surfaces. In: Nakashima S, Maruyama S, Brack A, Windley BF (eds) Geochemistry and the Origin of Life. Universal Academy Press, Tokyo, pp 51–60
Brubaker GR, Sakkab NY (1974) Models for metal-protein interaction: copper (II) complexes with some substituted cyclic dipeptides. Bioinorg Chem 3:243–260
Bujdak J, Rode B (1996) Clays and catalysis of prebiotic peptide bond formation. Acta Univ Carol Geol 38:139–143
Chung NM, Lohrmann R, Orgel LE, Rabinowitz J (1971) The mechanism of the trimetaphosphate-induced peptide synthesis. Tetrahedron 27:1205–1210
Chyba C, Sagan C (1992) Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: an inventory for the origins of life. Nature 355:125–32
Coumou D, Driesner T, Heinrich CA (2008) The structure and dynamics of mid-ocean ridge hydrothermal systems. Science 321:1825–1828
Cronin JR, Long DA, Truscott TG (1971) Peptide kinetics. Part 12. - The effect of Zn (II) on the reaction of some glycine-containing dipeptides and diketopiperazines at pH 5.6 and 368.2 K. Trans Faraday Soc 67:2096–2100
Datta SP, Rabin BR (1956) Chelation of metal ions by dipeptides and related compounds. Biochim Biophys Acta 19:572–574
de Duve C, Miller SL (1991) Two-dimensional life? Proc Natl Acad Sci USA 88:10014–10017
Dobry A, Fruton JS, Sturtevant JM (1952) Thermodynamics of hydrolysis of peptide bonds. J Biol Chem 195:149–54
Domareva NV, Kotova DL, Krysanova TA, Cherenkova YA (2004) Effect of temperature on the exchange equilibrium in the histidine-H-form KU-2 × 8 sulfocationite system. Russ J Phys Chem 78:1470–1473
Elderfield H, Schultz A (1996) Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Ann Rev Earth Planet Sci 24:191–224
Ferris JP, Hill AR, Liu R, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61
Flegmann AW, Scholefield D (1978) Thermodynamics of peptide bond formation at clay mineral surfaces. J Mol Evol 12:101–112
Flegmann AW, Tattersall R (1979) Energetics of peptide bond formation at elevated temperatures. J Mol Evol 12:349–55
Flores JJ, Leckie JO (1973) Peptide formation mediated by cyanate. Nature 244:435–437
Fox SW, Harada K (1958) Thermal copolymerization of amino acids to a product resembling protein. Science 128:1214
Gaines SM, Bada JL (1988) Aspartame decomposition and epimerization in the diketopiperazine and dipeptide products as a function of pH and temperature. J Org Chem 53:2757–2764
Grzegorczyk DS, Carta G (1996) Adsorption of amino acids on porous polymeric adsorbents – I. Equilibrium. Chem Eng Sci 51:807–817
Hay RW, Morris PJ (1976) Metal ion-promoted hydrolysis of amino acid esters and peptides. Met Ions Biol Syst 5:173–243
Hazen RM, Filley TR, Goodfriend GA (2001) Selective adsorption of L- and D-amino acids on calcite: implications for biochemical homochirality. Proc Nat Acad Sci USA 98:5487–5490
Hennet RJC, Holm NG, Engel MH (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: a perpetual phenomenon? Naturwiss 79:361–365
Hill JR. AR, Böhler C, Orgel LE (1998) Polymerization on the rocks: negatively-charged-amino acids. Origins of Life Evol Biosph 28:235–243
Huber C, Wächtershäuser G (1998) Peptides by activation of amino acids with CO on (Ni,Fe)S Surfaces: Implications for the origin of life. Science 281:670–672
Huber C, Wächtershäuser G (2006) α-Hydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science 314:630–632
Imai E, Honda H, Hatori K, Brack A, Matsuno K (1999a) Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 283:831–833
Imai E, Honda H, Hatori K, Matsuno K (1999b) Autocatalytic synthesis of oligoglycine in a simulated submarine hydrothermal system. Origins of Life Evol Biosph 29:249–259
Islam MN, Kaneko T, Kobayashi K (2003) Reaction of amino acids in a supercritical water-flow reactor simulating submarine hydrothermal systems. Bull Chem Soc Jpn 76:1171–1178
Kadko D, Butterfield DA (1998) The relationship of hydrothermal fluid composition and crustal residence time to the maturity of vent fields on the Juan de Fuca Ridge. Geochim Cosmochim Acta 62:1521–1533
Kahne D, Still WC (1988) Hydrolysis of a peptide bond in neutral water. J Am Chem Soc 110:7529–7534
Kasting JF, Ackerman TP (1986) Climatic consequences of very high carbon dioxide levels in the Earth's early atmosphere. Science 234:1383–1385
Kozai T, Hayashi N, Ikeda Y (1975) Ring-opening polymerization of piperazine-2, 5-dione in the presence of aqueous ammonia. Kobunshi Ronbunshu Eng Ed 4:64–70
Lahav N, Chang S (1976) The possible role of solid surface area in condensation reactions during chemical evolution: reevaluation. J Mol Evol 8:357–80
Lahav N, White D, Chang S (1978) Peptide formation in the prebiotic era: thermal condensation of glycine in fluctuating clay environments. Science 201:67–69
Lambert JF (2008) Adsorption and Polymerization of Amino Acids on Mineral Surfaces: A Review. Origins of Life Evol. Biospheres 38:211–242
Leman L, Orgel L, Ghadiri MR (2004) Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306:283–286
Lemke KH (2003) Peptide synthesis under simulated deep-sea hydrothermal conditions, Ph.D. Dissertation, Stanford University
Li J, Wang X, Klein MT, Brill TB (2002) Spectroscopy of hydrothermal reactions, 19: pH and salt dependence of decarboxylation of α-alanine at 280–330°C in an FT-IR spectroscopy flow reactor. Int J Chem Kinetics 34:271–277
Li J, Brill TB (2003a) Spectroscopy of hydrothermal reactions, Part 26: Kinetics of decarboxylation of aliphatic amino acids and comparison with the rates of racemization. Int J Chem Kinetics 35:602–610
Li J, Brill TB (2003b) Spectroscopy of hydrothermal reactions. 27. Simultaneous determination of hydrolysis rate constants of glycylglycine to glycine and glycylglycine-diketopiperazine equilibrium constants at 310–330°C and 275 bar. J Phys Chem A 107:8575–8577
Li J, Brill TB (2003c) Spectroscopy of hydrothermal reactions 25: Kinetics of the decarboxylation of protein amino acids and the effect of side chains on hydrothermal stability. J Phys Chem A 107:5987–5992
Liu R, Orgel LE (1998a) Polymerization of amino acids in aqueous solution. Origins of Life Evol Biosph 28:47–60
Liu R, Orgel LE (1998b) Polymerization on the rocks: β-amino acids and arginine. Origins of Life Evol Biosph 28:245–257
Long DA, Truscott TG, Cronin JR, Lee RG (1971) Peptide kinetics Part 11. Influence of divalent metal ions on rate of reaction of glycyl-glycine in the pH ranges 0.3–1.0 and 3.8–6.0. Trans Faraday Soc 67:1094–1103
Marshall WL (1994) Hydrothermal synthesis of amino acids. Geochim Cosmochim Acta 58:2099–2106
Martin RB (1998) Free energies and equilibria of peptide bond hydrolysis and formation. Biopolymers 45:351–353
Maurel M, Orgel LE (2000) Oligomerization of α-thioglutamic acid. Origins of Life Evol Biosph 30:423–430
Meggy AB (1953) Glycine peptides. I. The polymerization of 2,5-piperazinedione at 180°. J Chem Soc851–855
Meggy AB (1956) Glycine peptides. II. Heat and entropy of formation of the peptide bond in polyglycine. J Chem Soc1444–1454
Meng M, Stievano L, Lambert J (2004) Adsorption and thermal condensation mechanisms of amino acids on oxide supports. 1. Glycine on silica. Langmuir 20:914–923
Miller SL, Orgel LE (1974) The Origin of Life on the Earth. Prentice-Hall, Englewood Cliffs
Mita H, Nomoto S, Terasaki M, Shimoyama A, Yamamoto Y (2005) Prebiotic formation of polyamino acids in molten urea. Int J Astrobiol 4:145–154
Morowitz HJ, Kostelnik JD, Yang J, Cody GD (2000) The origin of intermediary metabolism. Proc Nat Acad Sci USA 97:7704–7708
Nagayama M, Takaoka O, Inomata K, Yamagata Y (1990) Diketopiperazine-mediated peptide formation in aqueous solution. Origins of Life Evol Biosph 20:249–57
Nissenbaum A (1976) Scavenging of soluble organic matter from the prebiotic oceans. Origins of Life 7:413–16
Ogata Y, Imai E, Honda H, Hatori K, Matsuno K (2000) Hydrothermal circulation of seawater through hot vents and contribution of interface chemistry to prebiotic synthesis. Origins of Life Evol Biosph 30:527–537
Orgel LE (1998) Polymerization on the rocks: theoretical introduction. Origins of Life Evol Biosphere 28:227–234
Oró J, Guidry CL (1960) A novel synthesis of polypeptides. Nature 186:156–157
Perrin DD (1965) Dissociation constants of organic bases in aqueous solution. Butterworths, London
Porter TL, Eastman MP, Hagerman ME, Price LB, Shand RF (1998) Site-specific prebiotic oligomerization reactions of glycine on the surface of hectorite. J Mol Evol 47:373–377
Qian Y, Engel MH, Macko SA, Carpenter S, Deming JW (1993) Kinetics of peptide hydrolysis and amino acid decomposition at high temperature. Geochim Cosmochim Acta 50:813–823
Radzicka A, Wolfenden R (1996) Rates of uncatalyzed peptide bond hydrolysis in neutral solution and the transition state affinities of proteases. J Am Chem Soc 118:6105–6109
Rode BM, Son HL, Suwannachot Y, Bujdak J (1999) The combination of salt induced peptide formation reaction and clay catalysis: a way to higher peptides under primitive earth conditions. Orig Life Evol Biosph 29:273–286
Sato N, Daimon H, Fujie K (2002) Decomposition of glycine in high temperature and high pressure water. Kagaku Kogaku Robunshu 28:113–117
Schwartz AW (1981) Chemical evolution - the genesis of the first organic compounds. In: Duursma EK, Dawson R (eds) Marine Organic Chemistry. Elsevier Oceanography Series, Amsterdam, pp 7–30
Shapiro R (2006) Small molecule interactions were central to the origin of life. Q Rev Biol 81:105–125
Sharma VS, Mathur HB (1965) Thermodynamic properties of coordination complexes of transition metal ions with amino acids. Indian J Chem 3:475–479
Shock EL (1992a) Chemical environments of submarine hydrothermal systems. Orig Life Evol Biosph 22:67–107
Shock EL (1992b) Hydrothermal organic synthesis experiments. Orig Life Evol Biosph 22:135–46
Shock EL (1992c) Stability of peptides in high-temperature aqueous solutions. Geochim Cosmochim Acta 56:3481–3491
Shock EL, Amend JP, Zolotov MY (2000) The early Earth vs. the origin of life. In: Canup RM, Righter K (eds) Origin of the Earth and Moon. University of Arizona Press, Tucson, pp 527–543
Snider MJ, Wolfenden R (2000) The rate of spontaneous decarboxylation of amino acids. J Am Chem Soc 122:11507–11508
Sowerby SJ, Morth CM, Holm NG (2001) Effect of temperature on the adsorption of adenine. Astrobiol 1:481–487
Steinberg S, Bada JL (1981) Diketopiperazine formation during investigations of amino acid racemization in dipeptides. Science 213:544–545
Steinberg S, Bada JL (1983) Peptide decomposition in the neutral pH region via the formation of diketopiperazines. J Org Chem 48:2295–2298
Stribling R, Miller SL (1987) Energy yields for hydrogen cyanide and formaldehyde syntheses: the hydrogen cyanide and amino acid concentrations in the primitive ocean. Orig Life Evol Biosph 17:261–273
Taillades J, Collet H, Garrel L, Beuzelin I, Boiteau L, Choukroun H, Commeyras A (1999) N-carbamoyl amino acid solid-gas nitrosation by NO/NOx: A new route to oligopeptides via alpha-amino acid N-carboxyanhydride. Prebiotic implications. J Mol Evol 48:638–645
Tsukahara H, Imai E, Honda H, Hatori K, Matsuno K (2002) Prebiotic oligomerization on or inside lipid vesicles in hydrothermal environments. Orig Life Evol Biosph 32:13–21
Turekian KK, Cochran JK (1986) Flow rates and reaction rates in the Galapagos Rise Spreading Center Hydrothermal System as inferred from 228Ra/226Ra in vesicomyid clam shells. Proc Natl Acad Sci USA 83:6241–6244
White RH (1984) Hydrolytic stability of biomolecules at high temperatures and its implication for life at 250 degrees C. Nature 310:430–432
Yanagawa H, Nishizawa M, Kojima K (1984) A possible prebiotic peptide formation from glycinamide and related compounds. Orig Life 14:267–272
Yanagawa H, Kojima K (1985) Thermophilic microspheres of peptide–like polymers and silicates formed at 250 degrees C. J Biochem 97:1521–4
Zaia DAM (2004) A review of adsorption of amino acids on minerals: Was it important for origin of life? Amino Acids 27:113–118
Zhao M, Bada JL (1995) Determination of alpha-dialkylamino acids and their enantiomers in geological samples by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. J Chrom A 690:55–63
Zhang Y, Zindler A (1993) Distribution and evolution of carbon and nitrogen in Earth. Earth Planet Sci Letts 117:331–345
Acknowledgements
The authors would like to thank Dr. John H. Chalmers for assistance in the laboratory and Professor Joris Gieskes and Dr. Evan Solomon for discussions about the dynamics of hydrothermal vent systems. This work was supported by the NASA Specialized Center of Research and Training (NSCORT) in Exobiology and a grant from the UCSD Academic Senate committee on Research, and in part by an appointment to the NASA Postdoctoral Program at the Jet Propulsion Laboratory, California Institute of Technology, administered by Oak Ridge Associated Universities through a contract with NASA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cleaves, H.J., Aubrey, A.D. & Bada, J.L. An Evaluation of the Critical Parameters for Abiotic Peptide Synthesis in Submarine Hydrothermal Systems. Orig Life Evol Biosph 39, 109–126 (2009). https://doi.org/10.1007/s11084-008-9154-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-008-9154-1