Abstract
Glycolaldehyde and dl-glyceraldehyde reacted in a water-buffered solution under mildly acidic conditions and in the presence of chiral dipeptide catalysts produced pentose sugars whose configuration is affected by the chirality of the catalyst. The chiral effect was found to vary between catalysts and to be largest for di-valine. Lyxose, arabinose, ribose and xylose are formed in different amounts, whose relative proportions do not change significantly with the varying of conditions. With LL-peptide catalysts, ribose was the only pentose sugar to have a significant D-enantiomeric excess (ee) (≤44%), lyxose displayed an L-ee of ≤66%, arabinose a smaller L-ee of ≤8%, and xylose was about racemic. These data expand our previous findings for tetrose sugars and further substantiate the suggestion that interactions between simple molecules of prebiotic relevance on the early Earth might have included the transfer of chiral asymmetry and advanced molecular evolution.
Similar content being viewed by others
Introduction
To date, we have no understanding of the actual physico-chemical processes that allowed the emergence of biochemistry. There are, in fact, inherent difficulties in reaching any direct knowledge of emergent life processes, as they may have differed in their core molecular organization and function from even the simplest of extant life forms from which they are now too far removed. Furthermore, while reasonably acknowledging a prebiotic dominance to the chemistry of carbon in the appearance of life, it is not known to what extent early Earth physical and chemical environments affected this chemistry with influential contingencies. In spite of this inscrutability, there are working assumptions that can be reasonably made and would allow analytical inquiries into chemical processes of possible prebiotic significance.
A frequently discussed hypothesis is that the development of molecules and/or molecular traits that are currently indispensable to the execution of biochemical processes, such as the homochirality of most biomolecules, must have been also important for the molecular evolution that preceded extant life. Chiral homogeneity is unknown outside the biosphere and its origin in biochemistry is not known, however, a feature so pervasive as to define life processes has solicited many theories, which often parallel those addressing the origin of life (Pizzarello 2007).
One proposal, which follows closely on the idea of an evolutionary continuity between chemical and biochemical evolution (e.g., Oro 1961), is that biological homochirality was brought about by abiotic and prebiotic physico-chemical processes. The finding in meteorites of amino acids displaying enantiomeric excesses (ee) of the same configuration as found in terrestrial proteins (Cronin and Pizzarello 1997; see also Pizzarello et al. 2006, for a recent review) verifies the existence of molecular asymmetry in extraterrestrial samples and appears to support the hypothesis. It also offers a credible basis for the experimental investigation of whether these amino acids that, together with small peptides and amines are known to be active asymmetric catalysts in aqueous organic reactions (e.g., Paradowska et al. 2009), might have aided the progression of molecular asymmetry upon delivery to the early Earth.
With this aim, we previously studied a water-based prebiotic model of sugar syntheses from glycolaldehyde’s condensation under the catalytic influence of two non-racemic amino acids, alanine and isovaline; the first being a common protein amino acid and the latter the most abundant chiral amino acid found in meteorites (Pizzarello and Weber 2004). The method, carried out in buffered water solutions, was considered somewhat realistic for early Earth conditions and led to the production of tetrose sugars whose chiral configuration was affected significantly by the chirality of the amino acid catalyst; ee were the largest for threose produced by enantiomerically pure isovaline catalyst and decreased linearly with the decrease in the ee of the catalyst.
In a subsequent set of experiments, we tested the catalytic effect of di-, and tripeptides in the asymmetric syntheses of tetroses and found that the transfer of asymmetry by dipeptides was much more effective than by single amino acids, with ee in the tetrose products of over 80% (Weber and Pizzarello 2006). Erythrose was here the main recipient of the catalysts’ asymmetric effect instead of threose, a fact likely due to a change in the reaction pathway that could carry a prebiotic significance in view of the fact that erythrose and ribose have the same arrangement of HO-substituents at the chiral carbons along the alkyl chain.
We report here on the syntheses of pentose sugars obtained by the condensation of glycolaldehyde and dl-glyceraldehyde that employ the same general methodology and compare the asymmetric effect of several dipeptide catalysts, both homochiral and in scalemic mixtures of enantiomers.
Material and Methods
Peptides and other chemicals were obtained from commercial suppliers. Unless indicated otherwise in the tables and figures, the aqueous catalyzed reactions used 20 μmole of each glycolaldehyde and dl-glyceraldehyde that were reacted in the presence of 10 μmole of peptide catalyst in a 0.05 M sodium acetate buffer, pH 5.4 (0.40 ml total volume), at selected temperatures and time, in sealed ampules in vacuo. The reaction products were kept frozen at −80°C until analyzed. Control reactions in the absence of peptide catalysts were also conducted and produced trace amounts of all pentose products but no ee (yield was >1%).
For analysis of the reaction products, the reaction solution was brought to room temperature and a one third aliquot applied to a column filled with ~12 ml of cation exchange resin in hydrogen form (200–400 mesh, analytical grade, BIO-RAD Lab.). The column was eluted first with water and then with 2N ammonium hydroxide. Both eluates were then dried by rotary evaporator at 30°C. The dry water eluate residue was reacted with trifluoroacetic anhydride at 25°C to give the O-TFA derivatives of the sugars while the ammonium hydroxide residue was reacted first with 2-propanol (~2N HCl) for 24 h, at 25°C and, after drying of the alcohol, with trifluoroacetic anhydride, as above. Unused portions of the reaction solution were immediately frozen for repeat analyses.
Gas chromatographic separation was performed with a capillary column coated with a bonded cyclodextrin phase (Chirasil-Dex CB, Chrompack; 25 m × 0.25 mm, 0.25 mm thickness). The oven temperature program was: 70°C initial for 5 min, to 85°C @1° min−1, to 200°C @ 4°min−1, and 45 min of final temperature hold. Eight peaks were obtained for each pentose sugar, i.e., two sets of furanose and pyranose enantiomers, and the non-cyclic pentoses were absent or gave very small peaks. Also observed were the peaks of unreacted glyceraldehyde and glycolaldehyde dimers as well as those of later eluting compounds having mass spectra containing typical glyceraldehyde fragments. Of these, some showed significant ee but could not be identified. d-, and l- ee give mean values based on n integrated ion intensities, where n is ≥4 and included multiple specific ions selected from multiple runs (Fig. 1).
Example of the GC-MS separation of two sets of ribose enantiomers for a L-val-L-val catalyst sample relative to a standard; furanose and pyranose peaks, shown in that elution order, are distinguished mainly by the presence or absence (respectively) of the fragment at m/z 407 (molecular ion minus O-TFA, minus CH2)
Results
The condensation of glycolaldehyde and dl-glyceraldehyde proceeded readily under the above experimental conditions, in fact, some pentose products can be seen even without the presence of a catalyst. In the presence of dipeptide catalysts, all four pentoses are synthesized and were found as a mix of furanose and pyranose forms, together with low amounts of tetroses and byproducts of unidentifiable dimers and trimers of both aldehydes but, as it appears from their mass spectra, mainly glyceraldehyde. Arabinose is the dominant sugar product, followed by lyxose, ribose and xylose and this order of abundance remains consistent with changing conditions (Fig. 2). On the other hand, ee change significantly between the pentose products, both in dimension and configuration relative to catalyst. Lyxose (Lyx) and ribose (Rib) are the main recipients of ee, which are much lower for arabinose (2–11%) and do not appear to be present for xylose (the chromatographic resolution of this sugar’s enantiomers was poor and allowed only an approximate estimate of their ee that, if present, would be ≤2%). Lyx showed the largest ee values, which can be as high as 66% and have the same configuration as the catalyst; Rib, although showing lower ee values of 18–47%, has the interesting distinction of being the only pentose product of these reactions with ee of the opposite configuration to the peptide catalyst, i.e., LL-peptides catalyze the production of d-ribose ee.
As Table 1 also shows, ee do not increase with the pentose yields and are somewhat higher for reactions conducted at room temperature. Dipeptides of branched amino acids appear to be better catalysts than those of unbranched alanine and their catalytic influence persists to a scalemic mix of 50% homochiral peptide.
Discussion
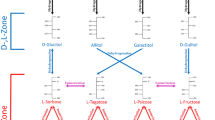
The variability in the distribution of preferred yields and ee observed between pentose sugars following the dipeptide-catalyzed reaction of glycolaldehyde and glyceraldehyde is at first puzzling. Because sugars do not follow a d- and l- classification that corresponds to the RS-stereochemical configurations of their first two chiral carbons, results can be better understood by comparing instead the spatial arrangements of the tetrose and pentose stereo families.
As Scheme 1 shows, it can be seen that the RS-stereochemical configurations of the first two chiral carbons of these sugars relate the d-ribose and l-lyxose, favored in the ll-di-valine syntheses, to d-erythrose that was shown to be favored by the same peptide in glycolaldehyde’s self-condensation (Weber and Pizzarello 2006). The ll-di-valine-unfavored l-ribose and d-lyxose are related to the unfavored L-erythrose as well, while arabinose and xylose, whose chiral distributions were not much affected by the catalytic syntheses, are likewise related to threose that was also less affected in the catalyzed glycolaldehyde condensation.
On this basis we considered plausible that the peptide-catalyzed syntheses of pentoses could follow a similar stereoselective pathway as that proposed for the tetroses, i.e., with the formation of an imidazolidinone ring. The reactions leading to formation of the imidazolidinone diasteromers from aldehydes and peptides are well established and the structure of the imidazolidinone products was confirmed by NMR and crystallographic analysis. (Roscic and Horvat 2006, and references therein).
The first step of this pathway is the attack of the amino group of a peptide (L-val-L-val, Scheme 2) on the aldehyde group of glycolaldehyde to give a carbinolamine intermediate. The carbinolamine then dehydrates yielding a Shiff base intermediate that undergoes intermolecular cyclization yielding two imidazolidinone diastereomers. These two diastereomers differ in the configuration at their C2-carbon that resides between the two nitrogens of the imidazolidinone ring. As shown, the S-isomer’s C2-hydrogen points below the ring, and the R-isomer’s hydrogen projects above the ring.
This participation of the imidazolidinone intermediate in sugar synthesis is supported by two findings in our previous study (Weber and Pizzarello 2006) that tetrose synthesis 1) yielded only racemic tetroses with gly-l-pro catalyst, a peptide lacking the hydrogen needed to form the imidazolidinone ring, and (2) had low stereoselectivity when carried out at 0°C with ll-di-valine, indicating a kinetic requirement for formation of an intermediate like the imidazolidinone. These syntheses also were found to be stereochemically controlled mainly by the peptide’s second amino acid residue, an observation that was difficult to explain without proposing an imidazolidinone intermediate.
This cyclization reaction is most likely stereoselective because studies have shown that the ratio of the S(C2) to R(C2)-imidazolidinone diastereomer is influenced by the chemical structure of the aldehyde (including sugars) and peptide reactants (Polt and Seebach 1989; Roscic an Horvat 2006). We expect that the intramolecular cyclization is followed by aldol condensation of the aldehyde group of dl-glyceraldehyde with the hydroxymethyl group attached to the C2-carbon of the imidazolidinone diastereomers yielding tetritol-imidazolidinones. In the final reaction the tetritol-imdiazolidinones hydrolyze to pentoses. The stereoselective behavior of the aldol condensation via these intermediates is shown by the preference of LL-peptides to catalyze an enantiomeric excess of d-ribose and l-lyxose and, vice versa, of d-peptides to catalyze an excess of l-ribose and d-lyxose. Although glyceraldehyde is also expected to react with dipeptides to give similar imidazolidinone intermediates, the subsequent aldol condensation of the imidazolidinone’s hydroxmethylene group with the aldehyde group of either a second glyceraldehyde or glycolaldehyde would yield branched sugar products that were not detected in our reactions.
Given the conformational complexity of the imidazolidinone intermediates and the aldol condensation transition state, it is difficult to identify the stereoselective mechanism that ties the asymmetry of the peptide catalyst to that of the sugar products. However, it is worthwhile to mention that imidazolidinone intermediates contain a secondary amine and a carboxylate group that, if effectively positioned, could act as proton donor-acceptor groups potentially capable of both enhancing the reactivity of the nucleophilic hydroxymethyl group involved in the aldol condensation and/or stabilizing the oxyanion on glyceraldehyde’s C1-carbon that forms in the aldol condensation transition state. Furthermore, the fact that the imidazolidinone forming cyclization reaction creates a chiral carbon adjacent to the hydroxymethyl group involved in the aldol condensation that sets the configuration of the C2, C3-pentose carbons, suggests that possibly the stereoselection of the S- or R-configuration of the C2-carbon of the imidazolidinone ring determines the stereoselectivity of sugar synthesis. In other words, possibly ll-dipeptides form S(C2)-imidazolidinone rings that react with glyceraldehyde to give a d-ribose enantiomeric excess, and dd-dipeptides form R(C2)-imidazolidinone rings that react with glyceraldehyde yielding a l-ribose enantiomeric excess. Some support for this idea comes from the observation that, as observed in tetrose pentose synthesis, the second amino acid and its size residue establishes the direction and degree of selection the imidazolidinone S/R diastereomer ratio (Polt and Seebach 1989).
As stated in the introduction, much is unknown about prebiotic processes and the chemical pathways that lead to biogenesis. However, if we accept the hypothesis that molecular evolution was important to the attainment of a complexity sufficient for life’s origin, then experimental models that include simple molecules that are important to extant biochemistry, can be produced abiotically, and are known to carry pre-biotic traits such as chiral biases, will be of particular interest. This is the case for the study we report here, where we show that the condensation of glycolaldehyde and DL-glyceraldehyde with non-racemic peptide catalysts is stereoselective and gives pentose sugars with significant ee. The results are intriguing because ribose is synthetically singled out to have a configuration opposite to that of the catalyst; i.e., d-ribose with ll-dipeptide catalysts, which has an obvious prebiotic resonance. These data expand our previous findings for tetrose sugars and further substantiate the suggestion that interactions between simple molecules of prebiotic relevance on the early Earth might have included the transfer of chiral asymmetry and advanced molecular evolution.
References
Cronin JR, Pizzarello S (1997) Enantiomeric excesses in meteoritic amino acids. Science 275:951–955
Oró J (1961) Comets and the formation of biochemical compounds on the primitive Earth. Nature 190:389–390
Paradowska J, Stodulski M, Mlynarski J (2009) Catalysts based on amino acids for asymmetric reactions in water. Angew. Chem. Int. Edit. 48:4288–4297
Pizzarello S (2007) The chemistry that precede life’s origin: a study guide from meteorites. Chem Biodivers 4:680–693
Pizzarello S, Weber AL (2004) Meteoritic amino acids as asymmetric catalysts. Science 303:1151
Pizzarello S, Cooper GW, Flynn GL (2006) The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In: Lauretta D, McSween HY (eds) Meteorites and the early Solar System II. University of Arizona Press, Tucson, pp 625–651
Polt R, Seebach D (1989) Stereoselective alkylation of glycine units in dipeptide derivatives: “chirality transfer” via a pivalaldehyde N, N-acetal center. J Am Chem Soc 11:2622–2632
Roscic M, Horvat S (2006) Transformations of bioactive peptides in the presence of sugars—characterization and stability studies of the adducts generated via the Maillard reaction. Bioorg Med Chem 14:4933–4943
Weber AL, Pizzarello S (2006) The peptide catalyzed stereospecific syntheses of tetroses: a possible model for prebiotic molecular evolution. Proc Nat Acad Sci USA 103:12713–12717
Acknowledgements
We thank Esther Varon for technical assistance in these studies and Ronald Breslow for reviewing the manuscript. This investigation was supported by grants from the National Aeronautics and Space Administration programs: Astrobiology and Exobiology (SP and AW), Cosmochemistry and Origins of the Solar System (SP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pizzarello, S., Weber, A.L. Stereoselective Syntheses of Pentose Sugars Under Realistic Prebiotic Conditions. Orig Life Evol Biosph 40, 3–10 (2010). https://doi.org/10.1007/s11084-009-9178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-009-9178-1