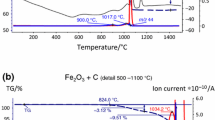
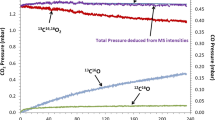
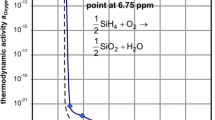
Mass spectrometry was used to study in-situ the role of volatile species in the oxidation of reference materials Cr, Cr2O3, Al, Al2O3, Si, SiO2, Fe and ferritic/martensitic steels (P91 and P92) at high temperatures. All samples were heated to 650°C at 1 atm in a mixture of Ar with 10–80% range H2O vapor. Oxidation times varied between 100 and 200 h. Cr(g), CrH(g), CrO(g), CrOH(g), CrO2(g), CrOOH(g) and Cr(OH)2(g) chromium-oxy-hydroxides species were identified during the corrosion of Cr oxidized in a steam atmosphere of Ar+80%H2O for 100 h. CrO(g), CrO3(g) and Cr(OH)6(g) species were present in mass loss of a Cr2O3 sample in similar conditions for 200 h of oxidation. However a mass gain was observed when Cr2O2(g) and CrO(OH)4(g) species were present. Simultaneously, thermogravimetric studies of the oxidation kinetics of the samples were made with one in-situ thermobalance. The P91 and P92 steels were studied with afore-mentioned techniques at the beginning of breakaway oxidation. During 100 h of oxidation at 650°C in Ar+10%H2O atmosphere: Cr(OH)6(g), CrOOH(g) and CrO2(OH)2(g) chromium oxy-hydroxides volatile species were detected in the P91 steel, and Cr(OH)2(g), Cr(OH)3(g), Cr(OH)4(g), CrO(OH)2(g) and CrO(OH)4(g), species in the P92 steel. The morphology/composition and structure of the oxidized steel samples were also characterized using SEM/EDAX and XRD techniques, respectively.













Similar content being viewed by others
References
Hald J., (2004). Materials at High Temperatures 21(1): 41
Khanna A. S., Rodríguez P., Gnanamoorthy J. B., (1986). Oxidation of Metals 26(3/4):171
Rowlands P. C., Garrett J. C. P., Hicks F. G., Lister S., Lloyd B., Twelves J. A., (1974) In: Holmes D. R., Hill R. B., Wyatt L. M., (eds) Corrosion of Steels in CO2. Proc. BNES, Reading, pp. 193
Khanna A. S., Kofstad P., (1990) International Conference Microscopy on Oxidation. Institute of Materials, London, p. 113
Asteman H., Segerdahl K., Svensson J. E., Johansson L. G., (2001). Materials Science Forum 369–372: 277
H. Asteman, J.-E. Svensson, and L.-G. Johansson, Oxidation of Metals 57 (3/4), 193 (2002)
Fryburg G. C., Miller R. A., Kohl F. J., Stearns C. A., (1977). Journal of the Electrochemical Society:Solid-State Science and Technology 124(11): 1738
Kim Y.-W., Belton G. R., (1974). Metailurgical Transactions 5: 1811
Bulewicz E. M., Padley P. J., (1971). Proceedings of the Royal Society London A 323: 377
Glemser O., Müller A., (1964). Zeitschrift fur anorganische and allgemeine Chemie 334: 151
Farber M., Srivastava R. D., (1973). Combustion Flame 20: 43
Glemser O., Müller A., Stocker U., (1964). Zeitschrift fur anorganische und allgemeine Chemie 344: 25
McDonald J. D., Margrave J. L., (1968). Journal of Inorganic Nuclear Chemistry 30: 665
Asteman H., Svensson J. E., Johansson L. G., Norell M., (1999). Oxidation of Metals 52 (1/2): 95
Asteman H., Segerdahl K., Svensson J.-E., Johansson L.-G., (2001). Materials Science Forum 369–372: 277
Graham H. C., Davies H. H., (1971). Journal of the American Ceramic Society 54: 89
Stearns C. A., Kohl F. J., Fryburg G. C., (1974). Journal of the Electrochemical Society 121: 945
Ebbinghaus B. B., (1993). Combustion and Flame 93: 119
Gulbransen E. A., Andrew K. F., (1957). Journal of the Electrochemical Society 104(6): 766
Kim Y.-W., Belton G. R., (1974). Metallurgical Transaction 5: 1811
Bulewicz E. M., Padley P. J., (1971). Proceeding of the Royal Society London Series A 323: 377
Farber M., Srivastava R. D., (1973). Combustion and Flame 20: 43
Gorokhov L. N., Milushin M. I., Emelyanov A. M., (1990). High Temperature Science 26: 395
Johnson J. R. T., Panas I., (1998). Inorganic Chemistry A 102: 10423
87-2334 @ 2001 JCPDS-International Centre for Diffraction Data
89-0597 @ 2001 JCPDS-International Centre for Diffraction Data
Acknowledgment
The authors acknowledge the financial support of the European Community under project N° ENK5-CT2002-00808-SUPERCOAT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Trujillo, F.J., Castañeda, S.I. Study by Means of the Mass Spectrometry of Volatile Species in the Oxidation of Cr, Cr2O3, Al, Al2O3, Si, SiO2, Fe and Ferritic/Martensitic Steel Samples at 923 K in Ar+(10 to 80%)H2O Vapor Atmosphere for New-Materials Design. Oxid Met 66, 231–251 (2006). https://doi.org/10.1007/s11085-006-9031-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-006-9031-0