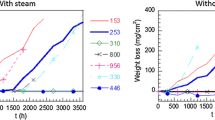
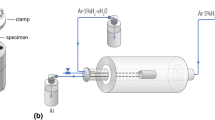
Abstract
Selective oxidation behavior of ferritic martensitic Fe–Cr base alloys, exposed in various atmospheres containing combinations of O2, CO2, and H2O, were studied at various temperatures relevant to oxy-fuel combustion. This paper begins with a discussion of the required Cr content to form a continuous external chromia scale on a simple binary Fe–Cr alloy exposed in oxygen or air based on experiments and calculations using the classic Wagner model. Then, the effects of the exposure environment and Cr content on the selective oxidation of Fe–Cr alloys are evaluated. Finally, the effects produced by alloying additions of Si, commonly present in various groups of commercially available ferritic steels, are described. The discussion compares the oxide scale formation on simple binary and ternary Fe–Cr base model alloys with that on several commercially available ferritic steels.

















Similar content being viewed by others
References
E. Essuman, G. H. Meier, J. Żurek, M. Hänsel, L. Singheiser, and W. J. Quadakkers, Scripta Materialia 57, 845 (2007).
J. Pirón Abellán, T. Olszewski, H. J. Penkalla, G. H. Meier, L. Singheiser, and W. J. Quadakkers, Materials at High Temperatures 26, 63 (2009).
W. J. Quadakkers, A. Elschner, W. Speier, and H. Nickel, Applied Surface Science 52, 171 (1991).
C. Wagner, Journal of the Electrochemical Society 99, 369 (1956).
R. A. Rapp, Acta Metallurgica 9, 730 (1961).
F. Gesmundo and F. Viani, Oxidation of Metals 25, 269 (1986).
C. Wagner, Zeitschrift für Elektrochemie 63, 772 (1959).
J. H. Swisher and E. T. Turkdogan, Transactions of the Metallurgical Society AIME 239, 426 (1967).
E. Fromm and E. Gebhardt, Gase und Kohlenstoff in Metallen (Springer Verlag, Berlin, 1976).
D. P. Whittle, G. C. Wood, D. J. Evans, and D. B. Scully, Acta Metallurgica 15, 1747 (1967).
C. S. Giggins and F. S. Pettit, Oxidation of Metals 14, 363 (1980).
C. T. Fujii and R. A. Meussner, Journal of the Electrochemical Society 114, 435 (1967).
J. Pirón Abellán, T. Olszewski, G. H. Meier, L. Singheiser, and W. J. Quadakkers, International Journal of Materials Research 101, 287 (2010).
A. Rahmel and J. Tobolski, Corrosion Science 5, 333 (1965).
C. T. Fujii and R. A. Meussner, Journal of the Electrochemical Society 111, 1215 (1964).
L. Tomlinson and N. J. Cory, Corrosion Science 29, 939 (1989).
M. H. B. Ani, T. Kodama, M. Ueda, K. Kawamura, and T. Maruyama, Materials Transactions 50, 256 (2009).
M. Michalik, M. Hänsel, J. Żurek, L. Singheiser, and W. J. Quadakkers, Materials at High Temperatures 22, 213 (2005).
S. Henry, J. Mougin, Y. Wouters, J.-P. Petit, and A. Galerie, Materials at High Temperatures 17, 231 (2000).
N. K. Othman, J. Zhang, and D. J. Young, Oxidation of Metals 73, 337 (2010).
J. Żurek, L. Niewolak, and W. J. Quadakkers, Unpublished Results, Forschungszentrum Jülich, FRG (2010).
G. R. Holcomb, Oxidation of Metals 69, 163 (2008).
I. G. Wright and R. B. Dooley, International Materials Reviews 55, 129 (2010).
W.J. Quadakkers, Unpublished Results, Forschungszentrum Jülich, FRG (2010).
A. M. Huntz, V. Bague, G. Beauple, C. Haut, C. Severac, P. Lecour, X. Longaygue, and F. Ropital, Applied Surface Science 207, 255 (2003).
B. Li and B. Gleeson, Oxidation of Metals 65, 101 (2006).
A. Kumar and D. L. Douglass, Oxidation of Metals 10, 1 (1976).
S. N. Basu and G. J. Yurek, Oxidation of Metals 36, 281 (1991).
J. F. Radavich, Corrosion 15, 613t (1959).
F. H. Stott, G. J. Gabriel, F. I. Wei, and G. C. Wood, Materials and Corrosion 38, 521 (1987).
V. Lepingle, G. Louis, D. Allué, B. Lefebvre, and B. Vandenberghe, Corrosion Science 50, 1011 (2008).
Acknowledgements
This work at UPitt was performed in support of the National Energy Technology Laboratory under RDS contract DE-AC26-04NT41817. The work at FZJ was supported by the German Ministry for Economics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meier, G.H., Jung, K., Mu, N. et al. Effect of Alloy Composition and Exposure Conditions on the Selective Oxidation Behavior of Ferritic Fe–Cr and Fe–Cr–X Alloys. Oxid Met 74, 319–340 (2010). https://doi.org/10.1007/s11085-010-9215-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-010-9215-5