Abstract
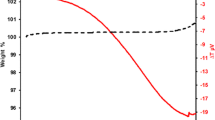
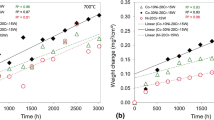
Numerous chromia-forming austenitic steels and nickel-base alloys contain chromium-rich strengthening precipitates, e.g. chromium-base carbides. During high temperature exposure the formation of the chromia base oxide scale results in chromium depletion in the alloy matrix and consequently in dissolution of the strengthening phase in the sub-surface zone. The present study describes the oxidation induced phase changes in the chromium depletion layer in case of alloy 625, a nickel base alloy in which the strengthening precipitates contain hardly any or only minor amounts of chromium. Specimens of alloy 625 were subjected to oxidation up to 1000 h at 900 and 1000 °C and analyzed in respect to oxide formation and microstructural changes using light optical microscopy, scanning electron microscopy, energy and wavelength dispersive analysis, glow discharge optical emission spectroscopy, and X-ray diffraction. In spite of the fact that the alloy precipitates δ-Ni3Nb and/or (Ni, Mo)6C contain only minor amounts of chromium, the oxidation induced chromium depletion results in formation of a wide sub-surface zone in which the precipitate phases are depleted. However, in parallel, substantial niobium diffusion occurs towards the alloy surface resulting in formation of a thin layer of δ-Ni3Nb phase adjacent to the alloy/oxide interface. By modeling phase equilibria and diffusion processes using Thermo-Calc and DICTRA it could be shown that the phase changes in the sub-scale zone are governed by the influence of alloy matrix chromium concentration on the thermodynamic activities of the other alloying elements, mainly niobium and carbon. The δ-phase depletion/enrichment process is caused by a decreasing niobium activity with decreasing chromium concentration whereas the (Ni,Mo)6C dissolution finds its cause in the increasing carbon activity with decreasing chromium content.

















Similar content being viewed by others
References
D. P. Whittle, D. J. Evans, D. B. Scully, and G. C. Wood, Acta Metallurgica 15, 1421 (1967).
B. D. Bastow, D. P. Whittle, and G. C. Wood, Oxidation of Metals 12, 413 (1978).
J. P. T. Vossen, P. Gawenda, K. Rahts, M. Rohrig, M. Schorr, and M. Schütze, Materials at High Temperatures 14, 387 (1997).
R. Bauer, M. Baccalaro, L. P. H. Jeurgens, M. Pohl, and E. J. Mittemeijer, Oxidation of Metals 69, 265 (2008).
H. E. Evans and A. T. Donaldson, Oxidation of Metals 50, 457 (1998).
W. J. Quadakkers and K. Bongartz, Werkstoffe und Korrosion 45, 232 (1994).
P. Kofstad, High Temperature Oxidation, (Elsevier Applied Science, London and New York, 1988).
C. Wagner, Journal of the Electrochemical Society 103, 571 (1956).
S. Kihara, J. B. Newkirk, A. Ohtomo, and Y. Saiga, Metallurgical Transactions A 11, 1019 (1980).
T. Sourmail, Materials Science and Technology 17, 1 (2001).
P. J. Ennis, W. J. Quadakkers, and H. Schuster, Journal de Physique IV 3, 979 (1993).
D. J. Young and B. Gleeson, Corrosion Science 44, 345 (2002).
D. Naumenko, V. Shemet, L. Singheiser, and W. J. Quadakkers, Journal of Materials Science 44, 1687 (2009).
W. G. Sloof and T. J. Nijdam, International Journal of Materials Research 100, 1318 (2009).
R. N. Durham, B. Gleeson, and D. J. Young, Oxidation of Metals 50, 139 (1998).
P. Krukovsky, K. Tadlya, A. Rybnikov, V. Kolarik, and W. Stamm, in Diffusion in Materials: Dimat 2004, Pt 1 and 2 (Trans Tech Publications Ltd, Zurich-Uetikon, 2005) p. 985.
D. Renusch, M. Schorr, and M. Schütze, Materials and Corrosion 59, 547 (2008).
K. V. Dahl, J. Hald, and A. Horsewell, in Diffusion in Solids and Liquids—MASS DIFFUSION, ed. A. Öchsner and J. Grácio (Trans Tech Publications Ltd, Stafa-Zurich, 2006), p. 73.
E. Y. Lee, D. M. Chartier, R. R. Biederman, and R. D. Sisson, Surface and Coatings Technology 32, 19 (1987).
J. A. Nesbitt, Oxidation of Metals 44, 309 (1995).
T. J. Nijdam and W. G. Sloof, Acta Materialia 56, 4972 (2008).
R. Cozar and A. Pineau, Metallurgical Transactions 4, 47 (1973).
M. D. Mathew, P. Parameswaran, and K. B. S. Rao, Materials Characterization 59, 2008 (508).
J. Froitzheim, G. H. Meier, L. Niewolak, P. Ennis, H. Hattendorf, L. Singheiser, and W. J. Quadakkers, Journal of Power Sources 178, 163 (2008).
P. D. Jablonski, C. J. Cowen, and J. S. Sears, Journal of Power Sources 195, 813 (2010).
Z. G. Yang, G. G. Xia, C. M. Wang, Z. M. Nie, J. Templeton, J. W. Stevenson, and P. Singh, Journal of Power Sources 183, 660 (2008).
V. Kochubey, H. Al Badairy, J. Le Coze, D. Naumenko, G. J. Tatlock, E. Wessel, and W. J. Quadakkers, Materials at High Temperatures 22, 461 (2005).
H. Nickel, W. J. Quadakkers, and L. Singheiser, Analytical and Bioanalytical Chemistry 374, 581 (2002).
B. Jansson, M. Schalin, M. Selleby, and B. Sundman, in Computer Software in Chemical and Extractive Metallurgy, ed. C. W. Bale and G. A. Irons (Canadian Inst Mining, Metallurgy and Petroleum, Montreal, 1993), p. 57.
A. Taylor and K. Sachs, Nature 169, 411 (1952).
M. J. Godden and J. Beech, Journal of Metals 21, A43 (1969).
T. M. Williams and J. M. Titchmarsh, Journal of Nuclear Materials 87, 398 (1979).
X. M. Guan and H. Q. Ye, Journal of Materials Science 15, 2935 (1980).
S. Hamar-Thibault, N. Valignat, and S. Lebaili, in X-Ray Optics and Microanalysis, ed. P. B. Kenway (Manchester, 1992), p. 189.
S. Lebaili, J. Ajao, and S. Hamarthibault, Journal of Alloys and Compounds 188, 87 (1992).
M. Sundararaman, L. Kumar, G. E. Prasad, P. Mukhopadhyay, and S. Banerjee, Metallurgical and Materials Transactions A 30, 41 (1999).
H. M. Tawancy, I. M. Allam, and N. M. Abbas, Journal of Materials Science Letters 9, 343 (1990).
E. Essuman, G. H. Meier, J. Zurek, M. Hansel, T. Norby, L. Singheiser, and W. J. Quadakkers, Corrosion Science 50, 1753 (2008).
P. Huczkowski, S. Ertl, J. Piron-Abellan, N. Christiansen, T. Hofler, V. Shemet, L. Singheiser, and W. J. Quadakkers, Materials at High Temperatures 22, 253 (2005).
W. E. Moddeman, S. M. Craven, and D. P. Kramer, Metallurgical Transactions A 17, 351 (1986).
F. Delaunay, C. Berthier, M. Lenglet, and J. M. Lameille, Mikrochimica Acta 132, 337 (2000).
E. N’Dah, M. P. Hierro, K. Borrero, and F. J. Perez, Oxidation of Metals 68, 9 (2007).
A. Borgenstam, A. Engstrom, L. Hoglund, and J. Agren, Journal of Phase Equilibria 21, 269 (2000).
L. S. Darken, Transactions of the American Institute of Mining and Metallurgical Engineers 180, 430 (1949).
H. Strandlund and H. Larsson, in Defects and Diffusion in Metals—an Annual Retrospective Vii, ed. D. J. Fisher (Trans Tech Publications Ltd, Zurich-Uetikon, 2004), p. 97.
P. Huczkowski, Forschungszentrum Jülich, Germany, Jülich, 2009, unpublished results.
M. Michalik, M. Hansel, J. Zurek, L. Singheiser, and W. J. Quadakkers, Materials at High Temperatures 22, 213 (2005).
V. B. Trindade, U. Krupp, P. E. G. Wagenhuber, and H. J. Christ, Materials and Corrosion 56, 785 (2005).
Acknowledgments
This research was supported by the German Research Foundation (DFG) under project SFB 561. The authors are also grateful to Mr. H. Cosler and Ms. A. Kick for carrying out the oxidation tests, Mr. V. Gutzeit and Mr. J. Bartsch for optical microscopy, Dr. E. Wessel for SEM investigations and Mr. M. Ziegner for XRD analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chyrkin, A., Huczkowski, P., Shemet, V. et al. Sub-Scale Depletion and Enrichment Processes During High Temperature Oxidation of the Nickel Base Alloy 625 in the Temperature Range 900–1000 °C. Oxid Met 75, 143–166 (2011). https://doi.org/10.1007/s11085-010-9225-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-010-9225-3