Abstract
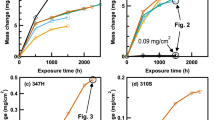
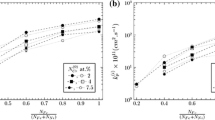
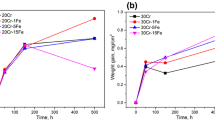
The oxidation behavior of a number of Fe–Cr- and Ni–Cr-based alloys was studied in atmospheres relevant to oxyfuel combustion at 650 °C. Oxidation was greatly enhanced in ferritic model alloys exposed in low p(O2) CO2 + 30%H2O and Ar + 30%H2O gases. Rapidly growing iron oxides appear to be porous and gas permeable. Transition from non-protective to protective oxidation occurs on alloys with higher Cr contents between 13.5 and 22 wt% in H2O. Excess oxygen, usually found in the actual oxyfuel combustion environments, disrupts the selective oxidation of Fe–Cr alloys by accelerating vaporization of early-formed Cr2O3 in combination with accelerated chromia growth induced by the H2O. Rapid Cr consumption leads to the nucleation and rapid growth of iron oxides. On the contrary, Ni–Cr alloys are less affected by the presence of H2O and excess O2. The difference between Fe–Cr and Ni–Cr alloys is not clear but is postulated to involve less acceleration of chromia growth by water vapor for the latter group of alloys.








Similar content being viewed by others
References
R. Viswanathan, J. Sarver and J. M. Tanzosh, Journal of Materials Engineering and Performance 15, 255 (2006).
G. R. Holcomb, Environmental Degradation—Steam Oxidation, in Power Plant Life Management and Performance Improvement, ed. J. E. Oakey, Chapt. 11 (Woodhead Publishing, Cambridge, 2011), ISBN: 978-1-84569-726-6.
J. P. Abellán, T. Olszewski, G. H. Meier, L. Singheiser and W. J. Quadakkers, International Journal of Materials Research 101, 287 (2010).
J. Shen, L. Zhou and T. Li, Oxidation of Metals 48, 347 (1997).
D. L. Douglass, P. Kofstad, A. Rahmel and G. C. Wood, Oxidation of Metals 45, 529 (1996).
K. Hilpert, D. Das, M. Miller, D. H. Peck and R. Weiss, Journal of the Electrochemical Society 143, 3642 (1996 ).
H. Asteman, J.-E. Svensson and L.-G. Johansson, Oxidation of Metals 57, 193 (2002).
G. R. Holcomb, Chromia Evaporation in Advanced Ultra-Supercritical Steam Boilers and Turbines, in Thermodynamics—Kinetics of Dynamic Systems, ed. J. C. Moreno-Piraján, Chapt. 9 (InTech, Rijeka, Croatia, 2011) ISBN: 978-953-307-318-7.
H. Asteman, J.-E. Svensson, L.-G. Johansson and M. Norell, Oxidation of Metals 52, 95 (1999).
A. Galerie, S. Henry, Y. Wouters, M. Mermoux, J.-P. Petit and L. Antoni, Materials at High Temperatures 22, 105 (2005).
C. T. Fujii and R. A. Meussner, Journal of the Electrochemical Society 111, 1215 (1964).
A. Galerie, Y. Wouters and M. Caillet, Material Science Forum 369–372, 237 (2001).
A. Rahmel and J. Tobolski, Corrosion Science 5, 333 (1965).
D. Renusch and M. Schütze, Materials at High Temperatures 22, 34 (2005).
E. Essuman, G. H. Meier, J. Żurek, M. Hänsel, L. Singheiser and W. J. Quadakkers, Scripta Materialia 57, 845 (2007).
M. H. B. Ani, T. Kodama, M. Ueda, K. Kawamura and T. Maruyama, Materials Transactions 50, 256 (2009).
M. Michalik, M. Hänsel, J. Żurek, L. Singheiser and W. J. Quadakkers, Materials at High Temperatures 22, 213 (2005).
S. Henry, J. Mougin, Y. Wouters, J.-P. Petit and A. Galerie, Materials at High Temperatures 17, 231 (2000).
N. K. Othman, J. Zhang and D. J. Young, Oxidation of Metals 73, 337 (2010).
G. H. Meier, K. Jung, N. Mu, N. M. Yanar, F. S. Pettit, J. P. Abellán, T. Olszewski, L. N. Hierro, W. J. Quadakkers and G. R. Holcomb, Oxidation of Metals 74, 319 (2010).
Materials Preparation Center at the U.S. Department of Energy’s Ames Laboratory; http://www.ameslab.gov/mpc. Accessed Feb 2012.
http://www.netl.doe.gov/. Accessed Feb 2012.
H. Davies and A. Dinsdale, Materials at High Temperatures 22, 15 (2005).
H. E. Evans, International Materials Review 40, 1 (1995).
M. Schütze, D. Renusch and M. Schorr, Corrosion Engineering, Science and Technology 39, 157 (2004).
A. Donchev, H. Fietzek, V. Kolarik, D. Renusch and M. Schütze, Materials at High Temperatures 22, 139 (2005).
B. A. Nevzorov, O. V. Starkov and N. G. Baranov, Zaschita Metallov 6, 349 (1970).
W. J. Quadakkers, P. J. Ennis, J. Żurek and M. Michalik, Materials at High Temperatures 22, 47 (2005).
W. J. Quadakkers, T. Olszewski, J. Piron-Abellán, V. Shemet and L. Singheiser, Materials Science Forum 696, 194 (2011).
T. Gheno, D. Monceau, J. Zhang and D. J. Young, Corrosion Science 53, 2767 (2011).
C. Anghel, E. Hörnlund, G. Hultquist and M. Limbäck, Applied Surface Science 233, 392 (2004).
C. S. Giggins and F. S. Pettit, Oxidation of Metals 14, 363 (1980).
C. Wagner, Journal of the Electrochemical Society 99, 369 (1956 ).
R. A. Rapp, Acta Metallurgica 9, 730 (1961).
F. Gesmundo and F. Viani, Oxidation of Metals 25, 269 (1986).
C. Wagner, Zeitschrift für Elektrochemie 63, 772 (1959).
M. Hänsel, W. J. Quadakkers and D. J. Young, Oxidation of Metals 59, 285 (2003).
A. Milewska, M. P. Hierro, J. A. Trilleros, F. J. Bolivar and F. J. Perez, Materials Science Forum 461–464, 321 (2004).
R. J. Ehlers, D. J. Young, E. J. Smaardijk, A. K. Tyagi, H. J. Penkalla, L. Singheiser and W. J. Quadakkers, Corrosion Science 48, 3428 (2006).
A. Rahmel, Corrosion Science 5, 815 (1965).
J. Zurek, D. J. Young, E. Essuman, M. Hänsel, H. J. Penkalla, L. Niewolek and W. J. Quadakkers, Materials Science and Engineering A 477, 259 (2008).
Acknowledgments
This work at University of Pittsburgh was performed in support of the National Energy Technology Laboratory’s ongoing research on Advanced Combustion under RES contract DE-FE0004000. The authors are most grateful to Prof. Shigenari Hayashi for help with the GD-OES measurements.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mu, N., Jung, K.Y., Yanar, N.M. et al. Water Vapor Effects on the Oxidation Behavior of Fe–Cr and Ni–Cr Alloys in Atmospheres Relevant to Oxy-fuel Combustion. Oxid Met 78, 221–237 (2012). https://doi.org/10.1007/s11085-012-9302-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-012-9302-x