Abstract
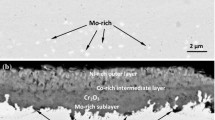
Commercially available high pressure tubing materials SS316 and Hastelloy-C276, containing a similar chromium content about 17 wt%, were internally pressurised by flowing supercritical CO2 at 20 MPa and heated externally, to 650 °C in the case of SS316, and 650–750 °C in the case of Hastelloy-C276. The stainless steel was found to be corroded by both external oxidation and internal carburisation. The external oxide was initially protective Cr-rich oxide, which was subsequently disrupted by faster growing Fe-rich oxide nodules, leading to loss of protection. In the case of Hastelloy-C276, the corrosion product was principally protective chromium oxide scale, although somewhat thicker regions of the same oxide developed at the site of micro-cracks in the metal surface. Very little carburisation of this alloy was observed up to at least 1000 h exposure. Differences in scaling behaviour are discussed in terms of chromium depletion through internal carbide precipitation.

















Similar content being viewed by others
References
C. S. Turchi, Z. Ma, T. W. Neises and M. J. Wagner, Journal of Solar Energy Engineering 135, 041007-1 (2013).
J. Pasch, T. Conboy, D. Fleming, and G. Rochau. Supercritical CO 2 Recompression Brayton Cycle: Completed Assembly Description. SANDIA REPORT, SAND2012-9546 (2012), p. 40.
V. Dostal, PhD Thesis, Massachusetts Institute of Technology (2004).
C. T. Fujii and R. A. Meussner, Journal of Electrochemical Society 114, 435 (1967).
F. S. Pettit, J. A. Goebel and G. W. Goward, Corrosion Science 9, 903 (1969).
F. Rouillard, G. Moine, M. Tabarant and J. C. Ruiz, Oxidation of Metals 77, 57 (2012).
F. Rouillard, G. Moine, L. Martinelli and J. C. Ruiz, Oxidation of Metals 77, 27 (2012).
H. E. McCoy, Corrosion 21, 84 (1965).
P. Promdirek, G. Lothongkum, S. Chandra-Ambhorn, Y. Wouters and A. Galerie, Oxidation of Metals 81, 315 (2014).
S. B. Newcomb, W. M. Stobbs and E. Metcalfe, Philosophical Transactions Royal Society A 319, 191 (1986).
W. M. Stobbs, S. B. Newcomb and E. Metcalfe, Philosophical Transactions Royal Society A 319, 219 (1986).
S. Bouhieda, F. Rouillard and K. Wolski, Materials at High Temperatures 29, 151 (2012).
J. Pirón-Abellán, T. Olszewski, H. J. Penkalla, G. H. Meier, L. Shingheiser and W. J. Quadakkers, Materials at High Temperatures 26, 63 (2009).
C. Gleave, J. M. Calvert, D. G. Lee and P. C. Rowlands, Proceedings of The Royal Society London A 379, 409 (1982).
L. Tan, M. Anderson, D. Taylor and T. R. Allen, Corrosion Science 53, 3273 (2011).
G. Cao, V. Firouzdor, K. Shridharan, M. Anderson and T. R. Allen, Corrosion Science 60, 246 (2012).
V. Firouzdor, K. Shridharan, G. Cao, M. Anderson and T. Y. R. Allen, Corrosion Science 69, 281 (2013).
T. Gheno, D. Monceau, J. Zhang and D. Young, Corrosion Science 53, 2767 (2011).
C. Wagner, Journal of The Electrochemical Society 99, 369 (1952).
D. Young, P. Huczkowski, T. Olszewski, L. Singheiser and W. J. Quadakkers, Corrosion Science 85, 1 (2014).
I. Wolf and H. J. Grabke, Solid State Communications 54, 5 (1985).
D. Young, T. D. Nguyen, P. Felter, J. Zhang and J. Cairney, Scripta Materialia 77, 29 (2014).
X. G. Zheng and D. J. Young, Oxidation of Metals 42, 163 (1994).
S. Bouhieda, F. Rouillard, V. Barnier and K. Wolski, Oxidation of Metals 80, 493 (2013).
T. W. Neises, M. J. Wagner, and A. K. Gray, in Proceedings of the 8th International Conference on Energy Sustainability Boston, Massachusetts (30 June–2 July, 2014).
NIDI Designers Handbook-Series No. 9004. High-Temperature Characteristics of Stainless Steel.
Haynes International, personal communication.
T. Furukawa, Y. Inagaki and M. Aritomi, Journal of Power and Energy Systems 4, 252 (2010).
F. Rouillard, F. Charton, and G. Moine, in Proceedings of SCO 2 Power Cycle Symposium 2009 (RPI, Troy, April 29–30, 2009), p. 7.
M. Hansel, C. A. Boddington and D. J. Young, Corrosion Science 45, 967 (2003).
D. J. Young, International Journal of Hydrogen Energy 32, 3763 (2007).
D. R. Holmes, R. B. Hill and L. M. Wyatt, in Corrosion of Steels in CO 2 , eds. D. R. Holmes, R. B. Hill and L. M. Wyatt (British Nuclear Energy Society—BENES, London, 1994).
FactSage 6.4 (Revised May 2013)—Thermochemical software and database. Thermfact/CRCT www.factsage.com
D. J. Young, High Temperature Oxidation and Corrosion of Metals. Elsevier Corrosion Series, 1st ed, (Elsevier, Amsterdam, 2008).
O. Kubaschewski, V. B. B. Alcock, and P. J. Spencer, Materials Thermochemistry. 6th edn revised (Pergamon Press, New York, 1993)
Acknowledgments
This project has been supported by the Australian Government through the Australian Renewable Energy Agency (ARENA). The Australian Government, through ARENA, is supporting Australian research and development in solar photovoltaic and concentrating solar power technologies to help solar power become cost competitive with other energy sources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olivares, R.I., Young, D.J., Marvig, P. et al. Alloys SS316 and Hastelloy-C276 in Supercritical CO2 at High Temperature. Oxid Met 84, 585–606 (2015). https://doi.org/10.1007/s11085-015-9589-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-015-9589-5