Abstract
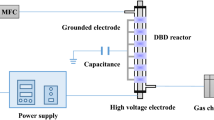
Despite a large interest in plasma-assisted catalytic technology (PACT), very little has been reported about the catalytic effects of different dielectric barriers on a dielectric barrier discharge (DBD) reaction. In the present study, Ca0.8Sr0.2TiO3, that possesses a high permittivity, was prepared by liquid phase sintering and used as a dielectric barrier in a DBD reactor to break CO2. The mechanical and dielectric properties of Ca0.8Sr0.2TiO3 were greatly enhanced by adding 0.5 wt.% Li2Si2O5. A DBD plasma was successfully generated by using this Ca0.8Sr0.2TiO3 as a dielectric barrier and 18.8% CO2 conversion was achieved with the residence time of 0.17 s at the frequency of 8 kHz, which was much higher than with those using an alumina or a silica glass barrier. It was found that the plasma power increased with the increasing of the permittivity, and finally very dense and strong microdischarges were initiated to decompose CO2.
Similar content being viewed by others
References
Eliasson B, Kogelschatz U (1991). IEEE Trans Plasma Sci 19:1063
Eliasson B, Hirth M, Kogelschatz U (1987). J Phys D: Appl Phys 20:1421
Sun W, Pashaie B, Dhali SK (1996). J Appl Phys 79:3438
Chang MB, Balbach JH, Rood MJ, Kushner MJ (1991). J Appl Phys 69:4409
Zheng G, Jiang J, Wu Y, Zhang R, Hou H (2003). Plasma Chem Plasma Process 23:59
Suib SL, Brock SL, Marquez M, Luo J, Matsumoto H, Hayashi Y (1998). J Phys Chem B 102:9661
US patent 5 474 747 (1995)
Ohgaki K, Nakano S, Matsubara T, Yamanaka S (1997). J Chem Eng Jpn 30:310
Brock SL, Marquez M, Suib SL, Hayashi Y, Matsumoto H (1998). J Catal 180:225
Wang J, Xia G, Huang A, Suib SL, Hayashi Y, Matsumoto H (1999). J Catal 185:152
Kraus M, Eliasson B, Kogelschatz U, Wokaun A (2001). Phys Chem Chem Phys 3:294
Kraus M, Egli W, Haffner K, Eliasson B, Kogelschatz U, Wokaun A (2002). Phys Chem Chem Phys 4:668
Matsumoto H, Tanabe S, Okitsu K, Hayashi Y, Suib SL (1999). Bull Chem Soc Jpn 72:2567
Schmidt-Szalowski K, Borucka A (1989). Plasma Chem Plasma Process 9:235
Drimal J, Gibalov VI, Samoylovich VG (1987). Czech J Phys B 37:1248
Drimal J, Kozlov KV, Gibalov VI, Samoylovich VG (1988). Czech J Phys B 38:159
Gibalov VI, Drimal J, Wronski M, Samoilovich VG (1991). Contrib Plasma Phys 31:89
Kogelschatz U, Eliasson B, Egli W (1997). J Phys IV, France 7:C4–47
Ball CJ, Begg BD, Cookson DJ, Thorogood GJ, Vance ER (1998). J Solid State Chem 139:238
Ceh M, Kolar D, Golic L (1987). J Solid State Chem 68:68
Qin S, Becerro AI, Seifert F, Gottsmann J, Jiang J (2000). J Mater Chem 10:1609
Howard CJ, Withers RL, Kennedy BJ (2001). J Solid State Chem 160:8
Yamanaka T, Hirai N, Komatsu Y (2002). Am Mineral 87:1183
Purwasasmita BS, Hoshi E, Kimura T (2001). J Ceram Soc Jpn 109:191
Kogelschatz U, Eliasson B, Egli W (1999). Pure Appl Chem 71:1819
Kogelschatz U (2003). Plasma Chem Plasma Process 23:1
Kogelschatz U, Salge J (2001). In: Hippler R, Pfau S, Schmidt M, Schoenbach KH (eds.) Low temperature plasma physics, Wiley-VCH Press, Berlin, pp 331–357
Xu X (2001). Thin Solid Films 390:237
Kappes T, Schiene W, Hammer T (2002). Proceedings 8th intern symposium on high pressure low temp. plasma chem., Hakone, pp 196–200
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, R., Tang, Q., Yin, S. et al. Preparation and Application of Ca0.8 Sr0.2 TiO3 for Plasma Activation of CO2 . Plasma Chem Plasma Process 26, 267–276 (2006). https://doi.org/10.1007/s11090-006-9002-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-006-9002-x